Open Science Repository Veterinary Medicine
doi: 10.7392/Research.70081919
Seroepidemiological Study of African Horse Sickness in Southern Ethiopia
Gizachew Hailegebreal Demissie DVM, Animal Health Researcher
ORCID 0000-0001-5202-7561, Worabe Agricultural Research Center, Ethiopia
Abstract
A cross-sectional study was carried out from November 2009 to mid of March 2010 to determine the seroprevalence of African horse sickness antibodies in the equine population of three selected districts - Damot Gale, Shashogo and Bonke Woredas - of the South Western Ethiopia. In total, 224 serum samples originating from 141 horses, 59 donkeys and 24 mules were collected. Indirect Enzyme-Linked Immunosorbent Assay (I-ELISA) configuration was employed to determine the presence of African horse sickness virus (AHSV) antibodies. The total apparent prevalence was found to be 33.04% (74/224), indicating the spread of AHS virus throughout the study areas with horses, donkeys and mules experiencing 36.17%, 25.42% and 33.33% of prevalence, respectively. In all districts, the distribution of African horse sickness was observed with the prevalence ranging from 25% to 37.5%. In this study, agroecology was one of hypothesized risk factors accordingly; the prevalence in the lowland was 25%, in midland 35% and 37.5% in the highland. All equines between the age group of 4 months to > 20 years were sampled in this study, with their prevalence ranging from 20% to 34.46%. Out of total 224 samples, 187 serum samples were from male and 37 serum samples from female with prevalence of 34.22% (64/187) and 27.03% (10/37), respectively. In all hypothesized risk factors, the seroprevalence of AHS was statistically insignificant (p > 0.05). The findings of this study, 33.04% seroprevalence of AHS in equines of the selected study areas, suggest that AHS virus is circulating throughout the study areas, regardless of the hypothesized risk factors. Therefore, this result warns a need for detail and further epidemiological study that should be carried out, as well as comprehensive investigations to assess the economic impacts of the disease, so that the appropriate control measures can be put in place to combat further spread.
Keywords: African horse sickness, Equines, Damot Gale, Shashogo, Bonke, seroprevalence.
Citation: Demissie, G. H. (2013). Seroepidemiological Study of African Horse Sickness in Southern Ethiopia. Open Science Repository Veterinary Medicine, Online(open-access), e70081919. doi:10.7392/Research.70081919
Received: December 19, 2012.
Accepted: January 16, 2013.
Published: January 21, 2013.
Copyright: © 2013 Demissie, G. H. Creative Commons Attribution 3.0 Unported License.
1. Introduction
African horse sickness (AHS) (peste equine africana, peste equine) is an infectious, non-contagious arthropod borne viral disease of equidae, caused by a double-stranded RNA Orbivirus belonging to the family Reoviridae, characterized by alterations in the respiratory and circulatory functions (OIE, 2008). AHS virus affects all species of equidae family (horses, mules, donkeys and zebras) and transmitted by a biting midge belonging to the cullicoides genus (Mellor, 1994). This devastating disease was listed by the World Organization for Animal Health (OIE) as endemic in sub-Saharan Africa (OIE, 2008). In the 1980s, AHS spreads outside its endemic region, reaching Spain and Portugal (Mac Lachlan, et al., 2007; OIE, 2008; Meiswinkel, et al., 2003).
The disease has both a seasonal (late summer/autumn) and a cyclical incidence with major epizootics in Southern Africa during warm-phase events (Meiswinkel, and Pawerska, 2003; OIE, 2008). Mortality due to AHS is related to the species of equidae affected and to the strain or serotype of the virus. At least two field vectors are involved: Cullicoides imicola and Cullicoides bolitinos. Among the equidae, horses are the most susceptible to AHS with a mortality rate of 50-95%, followed by mules with mortality around 50%. In enzootic regions of Africa, donkeys are very resistant to AHS and experience only sub-clinical infections. In European and Asian countries, however, donkeys are moderately susceptible and have a mortality rate of 10%. Zebras are also markedly resistant with no clinical signs, except fever, and may have extended viraemia (up to 40 days) (Baylis et al., 1999; Roy et al., 1994; Williams et al., 1998; OIE, 2008).
African horse sickness virus (AHSV) belongs to the family Reoviridae, genus Orbivirus (OIE, 2004). Like other Orbiviruses, AHSV virions are double layered, with their genomes composed of 10 double-stranded RNA (dsRNA) segments (Roy et al., 1994). There are nine serologically distinct AHS virus serotypes (AHSV-1 to AHSV-9) have been identified with no evidence of cross-neutralization among themselves (OIE, 2004; Baylis et al., 1999). The disease is confined to sub-saharan Africa, although periodic epizootics have caused severe outbreaks of the disease outside enzootic regions, i.e., North Africa, the Middle East, and Southern Europe (Williams et al., 1998; Torrecuadrada et al., 1999). The nine known virus serotypes of AHSV have been isolated from clinical cases of the disease in Kenya (Binepal et al., 1992).
The most important factor in the epizootology of AHS is the reservoir host. The existence of the reservoir of the infection is suggested by the fact that the disease passes from one season to another in a particular area. An outbreak mostly occurs during rainy seasons and quickly disappears during dry and cool periods before reappearing when wet and warm weather returns (Sinklair, 2006; Feseha, 1998).
According to the central statistical authority of Ethiopia (2009), there are 5.42 million donkeys, 1.78 million horses and 373,519 mules in Ethiopia. It has the largest equine population, probably with the highest density per square kilometer in the world (Feseha, 1998). Many factors can contribute to the poor performance of equines, among which viral diseases characterized by high morbidity and mortality rates are to be the first one, and African horse sickness is one of the viral diseases (OIE, 2004).
An important but often overlooked aspect is that, in most cases, donkeys and mules are reared by landless and marginal farmers and are the means of subsistence for millions in the least privileged parts of the world. These beasts of burden receive little care and are subjected to intensive work throughout their lives. Like most part of the country, in Gamo Gofa, Wolaita and Hadiya areas as well, the attention given to these animals is very poor despite their enormous role in the economy and they are subjected to arrays of manage mental constraints and diseases. African horse sickness has also been known for years. However, no systematically collected information is available pertinent to the disease in question.
Therefore, this work was conducted for the objective here under:
- To estimate the prevalence of African horse sickness in the selected Woredas of Gamo Gofa, Wolaita and Hadiya zones, Southern Ethiopia.
2. Literature review
2.1. Etiology
A virus in the Orbivirus genus of the family Reoviridae causes African horse sickness. The genus Orbivirus also includes bluetongue virus and epizootic hemorrhagic disease virus, which have similar morphological and biochemical properties with distinctive pathological and antigenic properties as well as host ranges. Like other Orbiviruses, AHSV virions are double layered, with their genomes composed of 10 double-stranded RNA (dsRNA) segments (Roy et al., 1994). The virion is an enveloped particle of a size around 70 nm (Purse et al., 2005; Ferhandey et al., 2007; Radostitis et al., 2007). There are nine serologically distinct AHS virus serotypes (AHSV-1 to AHSV-9) which have been identified, with no evidence of cross-neutralization among them (OIE, 2004; Roy et al., 1994). Serotype 9 is widespread in endemic regions, while serotypes 1 to 8 are found only in limited geographic areas. Serotype 9 has been responsible for the majority of African horse sickness outbreaks outside Africa (Sailleau et al., 2000; Coetzer et al., 2004; Mellor, P.S., 1994).
2.1.1. Persistence of the virus
According to Martinez-Torrecuadrada et al. (2005), Grubman and Lewis (1992) and Murphy et al. (1999), AHS virus is very stable outside the host and the virus has the following properties.
- An optional pH for survival of 7.0 - 8.5; the virus is sensitive to acid pH values but is relatively resistant to alkaline conditions
- Resistant to ether and other lipid solvents
- Relatively heat stable
- Can be stored for at least six months at 4°C
- Not destroyed by putrefaction and may retain infectivity in putrid blood for more than two years
- It does not contain lipid and is resistant to detergents.
Generally, the virus can be detected in blood for 4 days before and 2 days after clinical signs are first observed. Persistence of the viraemic state for up to 49 days has been observed in vaccinated horses (Ferhandey et al., 2007). It can survive in frozen but not salted meat. At pH value below 6.0, that is, at the sort of pH usually found in meat that has rigor mortis, the virus of AHS is inactivated quickly. It is generally accepted that vectors that become infected with an Orbivirus remain so far life. AHS virus multiplies and reaches a titer in Cullicoides imicola on the fifth day after ingestion of infected blood. This midge has been able to transmit infection to other horses 7 - 13 days after feeding on an infected horse. The shortest period between a midge biting an infected horse and the disease being seen later in another horse bitten by that midge could be from 12 to 16 days (Anon, 1998; Meiswinkel and Pawerska, 2003).
2.2. Epidemiology
2.2.1. Susceptible species
All members of the horse family (Equidae: horses, mules, donkeys and zebras) are susceptible, with horses generally experiencing severest disease and highest mortality rates (Coetzer et al., 2004). The most serious infections occur in horses and mules, which appear to be accidental hosts. Dogs are also susceptible. The disease does not affect humans. Zebras and donkeys rarely develop serious clinical signs (Baylis et al., 1999; Barard, B.J.H., 1994). Serological surveys in Africa apparently showed AHS antibodies in elephant sera, but this is now thought to have been due to the elephant sera reacting non-specifically in the complement fixation test used (Koekemoer et al., 2000; Murphy et al., 1999).
African horse sickness is endemic in sub-Saharan Central and East Africa. This disease sometimes spreads to Southern Africa and occasionally to Northern Africa (Feseha, 1998). An epidemic of AHS tends to occur at cyclic intervals, and is associated with drought followed by heavy rain. Although zebras are thought to be the natural reservoir hosts, horses, mules and donkeys can also develop viraemia sufficient to infect cullicoides. Most sources suggest that dogs do not play a significant role in the maintenance or spread of AHS (Barard, 1994; OIE, 2008).
2.2.2. Vectors
Midges are not simply mechanical vectors, they rather allow the replication of the virus in themselves before transmission. In fact there is no report of transovarial transmission so far. Cullicoides imicola is the vector responsible for the transmission of AHSV with in its enzootic area and during epizootics. Cullicoides bolitinos is also a vector of AHSV in southern Africa while the other Cullicoide species are unlikely to be important as they are unable to maintain the infection (Meiswinkel and Paweska, 2003). However Cullicoides varipenis, Cullicoides pulicaris and Cullicoides absoletus are competent and likely important vectors because of their ability to maintain infection over winter, as demonstrated in Portugal (Radostitis et al., 2007).
The abundance of midges can be predicted from measure of soil moisture content and land surface temperature. Midges breed in dump soils that are rich in organic material, such as irrigated pastures that provide soil moisture adequate for completion of the life cycle (at least 7 to 10 days) ( Mellor, 1994). Higher temperatures increase the rates of infection of midges, virogenesis within midges and transmission rate, but decrease midge longevity. Replication of AHSV in midges does not occur at temperatures less than 15°,C although midges continue to be active at 12°C. Midges can be transported by winds for up to 700 km (Radostitis et al., 2007). As the main means of spread of AHS virus is by biting midges, conditions that favor the presence of large populations of these insects are required for epidemics of the disease to occur. Favorable conditions are high temperatures and humidity after widespread rains (Coetzer et al., 2004). Cullicoides midges generally feed in the twilight periods after sunrise and sunset, on fine clear nights. In overcast and cloudy conditions or in cooler weather, biting activity will occur in late afternoon, before sunset, and in the mornings after sunrise. Analyses of meteorological conditions suggest that windborne spread of infected vectors may have been responsible for a number of outbreaks of AHS in Spain, around the Mediterranean and in the Middle East and India. Distances involved varied from 40 - 700 kilometers (Meiswinkel, and Pawerska, 2003; OIE, 2004).
2.2.3. Modes of transmission
AHS is not a contagious disease. It is transmitted between susceptible animals by midges of the genus Cullicoides. It is not spread by aerosol or direct contact between infected and non-infected animals (Mellor, 1994). Both Cullicoides imicola and Cullicoides bolitinos are known to transmit AHSV in the field; Cullicoides imicola appears to be the principal vector. Other species of Cullicoides may also been able to spread this virus (OIE, 2008).Transmission by insects other than midges is thought to be a minor source of infection. Virus replication occurs mainly in the lungs, spleen and lymph nodes. Although virus is present in urine, milk and other body secretions of infected animals, no transmission of disease by contact, inhalation or ingestion of these materials is known (Roy et al., 1994; Binepal et al., 1992).
2.3. Pathogenesis
There are a number of factors that decide the outcome in the horse that is bitten by a midge infected by AHS virus, including the virulence of the individual virus serotype and the immune status of the horse (Radostitis et al., 2007). After the virus is inoculated into the body, it is carried to the regional lymph nodes where it finds conditions favorable to its multiplication. Virus is released into the blood whereby it finds itself infecting the target organs, namely the lungs and other lymphoid tissues of the body. The viraemia is associated with the red blood cells and lasts for about four to eight days. By the third day after inoculation, the virus may be found in organs such as the spleen, lungs and pharynx, as well as most lymph nodes. The heart is not primary site for virus replication (Meiswinkel and Pawerska, 2003; Ferhandey et al., 2007).
2.4. Clinical signs and lesions
2.4.1. Incubation period
In experimental infections, the incubation period can range from 2 to 21 days. In natural infections, the incubation period appears to be approximately 3 to 5 days for the pulmonary form, 7 to 14 days for the cardiac form and 5 to 7 days for the mixed form, and 5 to 14 days for horse sickness fever (Grubman and Lewis, 1992; OIE, 2004)
According to Binepal et al., 1992, Coetzer et al., 2004, and Sinklair, 2006, there are four different forms of African horse sickness. These are:
- The peracute, pulmonary or “dun kop” form
- The subacute, cardiac or “din kop” form
- The acute or mixed form
- The horse sickness fever.
Symptomatic infections occur most often in horses and mules. The pulmonary and mixed forms usually predominate in susceptible populations of horses.
2.4.2. Pulmonary form
The pulmonary form of African horse sickness, also called “dun kop”, is characterized by an incubation period of 3 - 5 days. Acute fever of 40 - 42°C (104 - 107°F) for 1 - 2 days is followed by the sudden onset of sever respiratory distress. Infected animals often stand with forelegs spread, head extended and nostrils fully dilated. The conjunctiva is congested and the supraorbital fossa may be swollen. Other clinical signs may include tachypnea, forced expiration, profuse sweating, spasmodic coughing and frothy serofibrinous nasal exudates. Dyspnea usually progresses rapidly, and the animal often dies within a few hours after the respiratory signs appear. Recovery is rare and death, which may reach up to 95%, is due to anoxia (Radostitis et al., 2007; OIE, 2008; Kahn and Line, 2005).
At necropsy, there is pulmonary edema, which is especially visible in the interlobular spaces. The lungs are distended and heavy, and frothy fluid may be found in the trachea, bronchi and bronchioles with occasional pleural effusion. The thoracic lymph nodes may be edematous, and the gastric fundus congested. Cardiac lesions usually are not outstanding, although petechiae are found in the pericardium, and there is an increase in pericardial fluid. The abdominal viscera may be congested. A frothy exudate may ooze from the nostrils. The pulmonary form is the usual form in dogs (Kahn and Line, 2005; OIE, 2008).
2.4.3. Cardiac form
The cardiac form of AHS is a subacute disease with a longer incubation period (1 - 2 weeks) and a more protracted course than the acute respiratory form. The fever (39 - 41°C) last less than two week and is followed by swelling of the supraorbital fossa, which is pathognomonic. Swelling usually extends to the eyelids, facial tissues, neck, thorax, brisket and shoulders. Death (50 - 70%) usually occurs within one week from cardiac failure. It is important to note that no edema of the lower legs is observed. If the animal recovers, the swellings gradually subside over the next 3 to 8 days (OIE, 2008; Coetzer et al., 2004; Sinklair, M., 2006).
At necropsy, petechiae and ecchymoses on the epicardium and endocardium are prominent. The lungs are usually flaccid or slightly edematous. There are yellow, gelatinous infiltrations of the subcutaneous and intramuscular tissues, especially along the jugular veins and ligamentum nuchae. Other lesions include hydro-pericardium with up to 2 liters of fluid in the pericardial sac, myocarditis, hemorrhagic gastritis and petechiae on the peritoneum and ventral surface of the tongue (Sinklair, 2006; Binepal et al., 1992; Kahn and Line, 2005).
2.4.4. Mixed form
In the mixed form of African horse sickness, symptoms of both the pulmonary and cardiac forms are seen. In most cases, the cardiac form is subclinical and is followed by severe respiratory distress, occasionally; mild respiratory signs may be followed by edema and death from cardiac failure (Radostitis et al., 2007; OIE, 2008; Coetzer et al., 2004). The mixed pulmonary and cardiac form of African horse sickness is rarely diagnosed clinically, which is usually found in outbreaks, and is the most commonly diagnosed form at post-mortem (Kahn and Line, 2005).
2.4.5. Horse sickness fever
The fever form of AHS is a mild to subclinical infection; the incubation period varies from 4 to 14 days. The characteristic fever of 39- 40°C usually lasts for 3 to 8 days; morning remissions and afternoon exacerbations are often seen, and may be the only clinical sign observed. Other symptoms are generally mild and may include mild anorexia or depression, edema of the supraorbital fossae, congested mucous membranes and an increased heart rate. Almost all animals affected with this form recover (OIE, 2008; Coetzer et al., 2004; Sinklair, 2006; Binepal et al., 1992).
2.5. Diagnosis
2.5.1. Clinical diagnosis
African horse sickness should be suspected in animals with typical symptoms of the cardiac, pulmonary or mixed forms of the disease. The supraorbital swellings are particularly characteristic of the disease. The horse sickness form can be difficult to diagnose (Ferhandy et al., 2007; European Commission, 2002).
2.5.2. Samples for virus isolation
Blood sample
In live animals, blood samples collected into anticoagulant tubes should be taken for virus isolation. Success is most likely if these samples are collected in heparinized tubes from acutely sick animals in the early febrile stage of the disease (OIE, 2004; Sashi et al, 1981).
Tissue sample
From relatively fresh dead bodies or moribund animals, small pieces (5-10gram) of spleen, lung and lymph nodes will be collected, which are the samples of choice for AHS virus diagnosis. Samples will be kept at +4°C or in glycerol-saline solution till processed (Alexander et al, 1995; OIE, 2004).
2.5.3. Laboratory tests
In the endemic areas, clinical signs and lesions may lead to a tentative diagnosis; however, laboratory confirmation is essential for definitive diagnosis and determination of the serotype, which is important for control measures (Ferhandy et al., 2007).
Enzyme-Linked Immunosorbent Assay (ELISA)
Enzyme-Linked Immunosorbent Assay (ELISA) will be used to detect the presence of specific antibody against the AHS virus in the collected sera samples following the kit manufacturers protocol (OIE, 2004).
Cell culture
Direct isolation of AHS virus from the outbreak samples has been used successfully using Baby Hamster Kidney (BHK-2) and African Green Monkey Kidney (Vero) cell lines (Bremer, 1998).
Virus detection
Blood samples collected in heparin will be washed with distilled water with a 1:10 ratio, this is to hemolize the erythrocytes; centrifuge the washed blood. This procedure removes unwanted antibody, which could neutralize free virus and promotes the release of virus associated with the red blood cell membranes (Sashi et al, 1981; OIE, 2004).
When tissue samples, such as spleen, lung or lymph node are used, a 10% tissue suspension is prepared in phosphate buffered saline (PBS) or cell culture medium, containing antibiotics (penicillin + streptomycin). 0.5 ml supernatant of the already prepared inoculums (hemolyzed blood or processed tissue suspension) shall be seeded into cofluently grown cells of 25 cm² of tissue culture flask. After 30 - 45 minutes of absorption time of incubations at 37°C, the inoculated suspension will be discarded and the culture should be washed with 10 ml sterile PBS and the cell culture flasks will be re-filled with 7 ml of maintenance medium (MEM+5% Fetal Calf Serum) and incubated at 37°C with 5% CO2. A cythopathic effect (CPE) will appear between 3 to 8 days post-inoculation and occasionally conspicuous CPE shall to be negative (Sashi et al, 1981; Du plessis, 1999).
Virus neutralization test
Cell culture that showed CPE will be assayed for identification of the responsible serotype involved in the infection using the standard nine serotypes of AHS virus and sera following the procedure of virus neutralization test (OIE, 2004).
Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
Extraction of nucleic acids from spleen, lung or lymph node samples will be carried out using RNA extraction kit and the isolated RNA will be used for DNA synthesis following the recommended protocol of the DNA synthesis kit. Reverse-Transcription Polymerase Chain Reaction (RT-PCR) will be applied for the confirmation of virus and for the discrimination of the nine AHS virus serotype (Cetre-Sossah et al, 2008). Nine pairs of sequence specific primers, forward and backward, will be designed for each serotype of AHS virus based on the sequence, analysis from the database and PCR amplification will be done. The number of cycle, the temperature and time involved for the PCR reaction will be set based on the primers and length of the amplified sequence. Analysis of the PCR product is carried out by 1% agarose electrophoresis gel containing ethidium bromide. AHS-positive sample will be used as a positive control in one lane which has an approximate length of 1179 base-pair band and a negative control sample will be loaded in another lane. The different produced viral DNA band pattern will be read/compared using a standard 1 Kb plus ladder (marker) that will be loaded on a separate lane (Belen et al, 2008).
2.6. Differential diagnosis
The differential diagnosis includes equine viral arteritis, equine infectious anemia, Hendra virus infection, purpura haemorrhagica and equine piroplasmosis. In Africa, equine encephalitis virus, another Orbivirus transmitted by cullicoides, causes a syndrome resembling horse sickness fever. Toxins, anthrax and other causes of sudden death, as well as disease that result in severe respiratory distress, should also be considered (Binepal et al., 1992; Coetzer et al., 2004; Sinklair, 2006).
2.7. Treatment, prevention and controls
There is no specific treatment for African horse sickness. Supportive care and treatment of complications of the disease should be provided (Radostitis et al., 2007).
Generally, according to Binepal et al., 1992, Kahn and Line, 2005, and Sinklair, 2006, control of AHS relies on effective quarantine and movement controls to stop the spread of infection, animal management to limit vector exposure, establishing immunity by vaccination.
2.7.1. Vector control
In the quest to prevent AHS transmission to horses, one has to consider the life cycle and typical habits of the midges. They are blood suckers and are drawn to their host - the horse, by the warmth emitted by that animal, the CO2 expired in its breath and the horse’s smell, which is always present in equine environments (Coetzer et al., 2004). Protection of horses has to be focused on preventing the midges from entering stables (to be able to bite the horses) or by confusing them so that the midges can not find the horses. A stable which has effective mosquito-proof mesh applied to all apertures should prove successful (OIE, 2008).
Whenever possible, all equines should be stabled in insect-proof housing. At a minimum, stabling from dusk to dawn, the period when cullicoides are most active, is recommended. Vector control measures such as modifications of cullicoides breeding areas, insect repellents, and targeted applications of insecticides or larvicides may also be useful (Sinklair, 2006). The cullicoides midges predominantly bite on the top of horses and therefore physical barriers like blankets or face masks, etc. will prevent midge’s biting (Bremer, 1998).
2.7.2. Vaccination
When the disease first appears in an area, affected equines should be eliminated immediately, and the non-infected equines vaccinated with polyvalent vaccine and rested for a week. When the virus isolate has been typed, animals that received polyvalent vaccine should be revaccinated with the homologous vaccine. Vaccinated horses should be kept in insect-proof housing because vaccine failure may occur (OIE, 2008; Mac Lachlan, et al., 2007).
Important attributes of an effective AHS vaccine include the induction of a high level of protection against death and clinical disease. Vaccines can be used to induce immunity in susceptible animals or to produce a barrier of resistant animals. The aim must be to achieve and maintain a high level of population immunity. Three types of vaccines can be considered: inactivated (‘killed’), attenuated (‘live’) and recombinant virus vaccines, each of which is discussed below (OIE, 2004).
Inactivated vaccine
Inactivated virus vaccines for AHS do not revert to virulence, do not cause a significant viraemia in inoculated animals, and do not re-assort with wild-type Orbivirus strains in the field. With inactivated vaccines, it may be possible to differentiate antibody elicited by the vaccine that resulting from infection with an active virus, which would allow free international movement of equines (OIE, 2008).
The first inactivated AHS virus vaccines were prepared by adding formalin to infect horse tissue emulsions and have been used experimentally since 1929. More recently, this technique has been superseded by production of inactivated AHS virus vaccines using purified formalin-treated virus prepared in cell culture on an industrial scale (Barard, 1994).
Attenuated vaccine
Vaccination with attenuated monovalent serotype AHS vaccine produces a solid immunity that probably lasts indefinitely. In Spain, about 10% of animals immunized for the first time with an attenuated monovalent AHS virus serotype 4 failed to seroconvert. However, at least some animals that failed to respond serologically were resistant to challenge infection (OIE, 2004).
Vaccination with an attenuated multivalent vaccine (containing AHS serotypes 1-6) produced a neutralizing antibody response to all 6 serotypes and demonstrated that a higher overall titer could be induced in most horses by multiple immunizations. Furthermore, some horses failed to respond to one or more of these serotypes despite the numerous immunizations and no cross protection against serotypes 7-9 was observed. The inability to respond to all virus strains in a polyvalent vaccine is thought to be due to ‘antigenic competition’ or over attenuation of vaccine strains. For these reasons, annual vaccination of horses in endemic AHS areas is advocated. In South Africa, two quadrivalent vaccines are produced; one containing serotypes 1, 3, 4 and 5; the other serotypes 2, 6, 7 and 8. most recently, polyvalent and later, monovalent (AHS-4) attenuated virus vaccines from South Africa have been used during the 1987 through 1990 AHS outbreaks in Spain (Barard, 1994; Martinez-Torrecaudrada et al., 2005).
Recombinant vaccines
Work on vaccines incorporating the virus proteins VP2, VP3, VP4 and VP7 is being done. AHSV serotype 4 outer capsid proteins VP2 and VP5 plus inner capsid protein Vp7, derived from single and recombinant expression vectors have been used in different combination to immunize horses (Maree, 2005).
2.8. Economic importance and zoonoses
In a fully susceptible horse population, the effect of AHS can be devastating, because up to 95% mortality can be expected (MAF, 1991). The serious nature of the disease for equines is compounded by tremendous problem of eradication: vaccination reduces the ravages of horse sickness, but even when practical on a wide scale it cannot eradicate the disease because the infection is insect borne, and uncontrolled hosts provide a reservoir of infection (Radostitis et al., 2005).
Very rarely, AHS can be zoonotic. The first evidence of this came when laboratory workers exposed to the virus during vaccine manufacture developed encephalitis, chorioretinitis and disseminated intravascular coagulation (Anon, 1998; Murphy et al., 1999).
There is no evidence that humans can become infected with any field strain of viscerotropic AHSV, either through contact with naturally or experimentally infected animals or by virus manipulation in laboratories. However, certain neurotropic vaccine strains that may cause encephalitis and retinitis in humans following transnasal infections have been described (Anon, 1998; OIE, 2004).
2.9. World distribution and occurrence in Ethiopia
African horse sickness is endemic in sub-Saharan central and east Africa. The disease sometimes spreads to southern Africa and occasionally to northern Africa. All serotypes of AHS occur in Eastern and Southern Africa. Only AHS serotype 9 and 4 have been found in West Africa, from where they occasionally spread into countries surrounding the Mediterranean. It has occurred in Egypt and the Middle East, extending to Pakistan and India in the early 1960’s. Spread also occasionally occurs from North Africa to the Iberian Peninsula. This distribution is primarily dictated by the presence of the principal insect vector, Cullicoides imicola (Sinclair, 2006). AHS epizootics become less common in southern Africa during the latter half of 20th century, possibly due to diseases in free-ranging Zebra populations. Examples of outbreaks that have occurred outside Africa are in the Middle East (1959-1963), in Spain serotype 9 (1966), serotype 4 (1977-1990), and in Portugal serotype 4 (1989) (OIE, 2008).
The study of Aschalew et al. (2005) revealed the presence of two serotypes of AHSV in Ethiopia (serotypes 6 and 9). Isolation and identification of a serotype of AHSV other than serotype 9 has not previously been shown in Ethiopia. Isolation of serotype 6 and its presence in Ethiopia was first reported by Aschalew et al (2005). The virus could have been introduced into Ethiopian equines by wind-borne infected midges (Cullicoides) from endemic regions of Africa. The vectors were known to be wind-driven and migrate, carrying the virus over 700 km (Quinn et al., 2002).
In 2002–2003 Ethiopia faced serious and repeated outbreaks of AHS in different regions, including southern, western, central, and northern Ethiopia. The outbreak affected horses vaccinated with monovalent vaccines containing type 9 AHSV (AHS Vaccine, National Veterinary Institute, Debre Zeit, Ethiopia). It is well documented that in spite of its wide distribution, serotype 9 of AHSV has a lower virulence than other serotypes, killing few horses in enzootic areas (Seifert, 1996). The outbreak encountered in 2002–2003, however, resulted in high mortality (Aschalew et al., 2005).
3. Materials and Methods
3.1. Description of study area
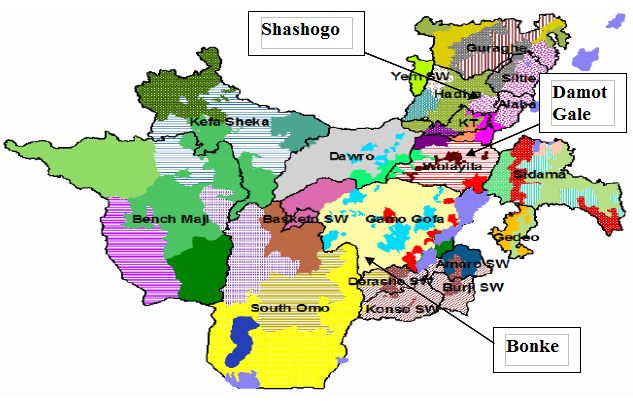
The study was carried out from November 2009 to mid of March 2010 in three selected Woredas, namely Bonke, Damot Gale and Shashogo of Gamo Gofa, Wolaita and Hadiya zones. The districts are located in the western part of southern nation’s nationalities and peoples region (SNNPR). Bonke, the district in Gamo Gofa zone, is situated 585 km south of Addis Ababa and 312 km west of Hawassa. The altitude of the area is between 950 - 2500 m above sea level. The mean annual temperature and rain fall of the area is 24.08°C and 1180 mm respectively. Damot Gale, the district in Wolaita, lies 380 km south of Addis Ababa and 135 km west of Hawassa. The area has an altitude range between 1600 - 2962 m above sea level and an average annual rainfall and temperature of the area is 1200 mm and 21°C, respectively. The third study district Shashogo is in Hadiya zone, located 289 km south of Addis Ababa and 217 km west of Hawassa. The area has an altitude of 1800 - 2000m above sea level with the mean annual temperature and rainfall of 19.5°C and 1200 mm, respectively. The predominant farming system in the areas is mixed livestock and crop production (SNNPRS ARDB, 2007).
3.2. Study design
For this particular study cross-sectional study design was conducted. Agroecologically representative districts were selected purposely and a two-stage cluster sampling technique was used to get the sample from the study population. Animals of the same village usually in communal grazing land were used to represent the primary sampling unit (epidemiological unit) and animals as the secondary units. All necessary epidemiological information was collected on individual animal bases using a structured questionnaire format (Annex).
3.3. Study animals
The study animals were equines, namely horses, donkeys and mules above 4 months of age from all ecological zones (lowland, midland and highland).
3.4. Sample size
A two stage cluster sampling technique was used to calculate the actual sample size, having the following parameters predetermined: CL= 90%, desired level of precision 5%, expected total clusters prevalence 23% (Kassa, 2006) and in between cluster variance 0.00279 (Thrusfield, 1995). Average number of equines per peasant association was estimated to be ten (10).
Then using the formula:
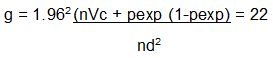
Where, n = herd size
VC = in between cluster variance
d = desired level of precision
pexp = expected prevalence
g = number of dusters needed
In order to get the total sample size:
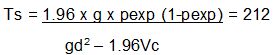
A total of 224 equines in 22 clusters were picked randomly from respective ecological zones.
3.5. Laboratory procedure
Serum was collected from selected sites and targeted species, approximately 5 ml of blood was collected using non-heparinized vacutainer tube and a serum was harvested from the clotted blood. Each serum sample was given an identification code and kept at -20°C until assayed in the laboratory. At the National Veterinary Institute (NVI), Enzyme-Linked Immunosorbent Assay (ELISA) was used to detect the presence of specific antibody against the AHS virus in the collected sera samples following manufactures protocol.
ELISA protocol
Preparation of reagents:
- Washing solution: dilute one part of the concentrate washing solution provided in the kit in 24 parts of distilled or deionized water (40 ml of concentrate solution and 960 ml of water). Once this solution is ready, it remains stable at +4°C.
- Preparation of controls (+) and (-): controls are ready to use and do not need any preparation. Dispense 100 μl of each.
- Preparation of conjugate: conjugate is ready to use and does not need any preparation.
- Preparation of substrate solution: mix one part of substrate with 9 parts of substrate buffer.
1. All reagents must be brought to room temperature before use
2. Dispense 100 µl of diluted samples into appropriate wells (dilution 1/5). Dispense 100 µl of positive control into two wells and 100 µl of negative control into two wells. Cover the plate and incubate for 1 hour at 37°C.
3. Washing steps:
- Throw out the content of the plate by a brusque turn over of the plate to avoid the possible mixture of the content from one well to another
- Dispense a volume of 300 µl of washing solution on each well
- Shake delicately the plate, avoiding the contamination between the wells
- Turn over the plate brusquely to empty the wells
- Repeat the process five times as indicated on the instructions of the kit
- Prior to empty the content of the last washing step, verify that the next reagent to be added to the plate is ready to use. Do not maintain the plate on dry more than strictly needed.
After the last step of washing shake the plate turned over on absorbent filter paper.
4. Wash 5 times as described before (3).
5. Add 100 µ / well of conjugate. Incubate for 30 minutes at 37°C.
6. Wash 5 times as described before (3).
7. Dispense 100 µl/well of substrate solution using a multi-channel pipette. Incubate for 10 minutes at room temperature.
8. Dispense 100 µl well of stop solution. Take care to avoid dispensing of bubbles.
9. Read at 405 nm using spectrophotometer.
10. Validation criteria:
- The ELISA test validation was checked for each plate based on two criteria set by the manufacturer for the mean optical density (OD) of the positive and negative control. The OD of the positive control must be less than 0.2 and the OD of the negative control must be higher than 1.0.
11. Interpretation
- Blocking percentage (BP) of each sample is calculated based on OD value applying the following formula:
- Samples showing BP value lower than 45% are considered to be negative for antibodies of AHS virus. Samples showing BP value higher than 50% are considered as positive for antibodies of AHS virus.
- Samples with BP value between 45% and 50% are considered doubtful and they must be retested. If the result is the same, another extraction must be made and tested 2 weeks later.
- Samples with BP value between 45% and 50% are considered doubtful and they must be retested. If the result is the same, another extraction must be made and tested 2 weeks later.
3.6. Data management and analysis
Recorded data were entered into Excel for the analysis of different attributable factors. The descriptive statistics was employed to quantify the results of seroprevalence of AHS antibodies. The association of potential risk factors such as different agroecological zones, species, sex and age were assessed using logistic regression employing windows STATA 9.0 (2006).
4. Results
Overall prevalence
A total of 224 serum samples were collected from three selected districts - Bonke, Damot Gale and Shashogo. Out of which 33.04 % (74/224) were found to be seropositive for AHSV antibody.
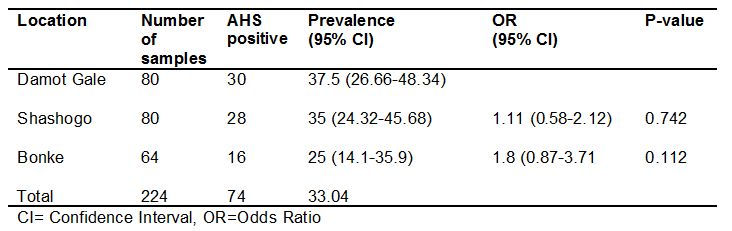
In all districts, the distribution of African horse sickness was observed with the prevalence ranging from 25% to 37.5% and no observable difference was seen statistically (p>0.05) (Table 1).
Table 1: Seroprevalence of AHS in relation to study districts.
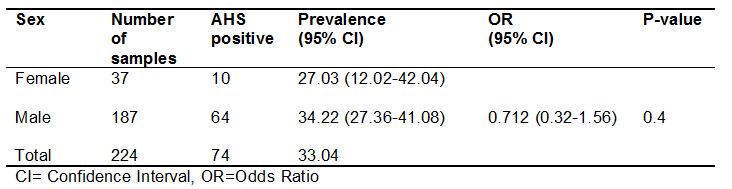
Out of total 224 samples, 187 serum samples were from male and 37 serum samples from female with prevalence of 34.22% (64/187) and 27.03% (10/37), respectively. Also, the prevalence that appeared between sex was not statistically significant (p>0.05) (Table 2).
Table 2: Seroprevalence of AHS in relation to sex.
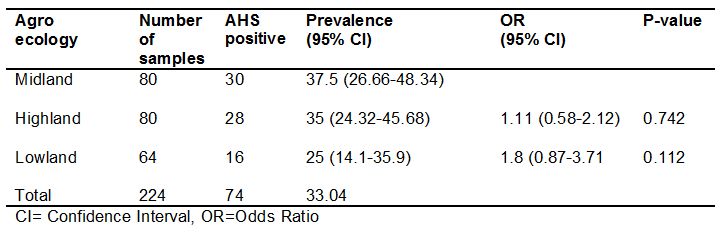
In this study agroecology was one of hypothesized risk factors; accordingly, 80 samples were from midland, 80 samples from highland and 64 samples from lowland. The prevalence in the lowland was 25%, in midland 35% and 37.5% in the highland. Once more here the observed difference in terms of agroecology were not different statistically (p>0.05) (Table 3).
Table 3: Seroprevalence of AHS in relation to agroecology.
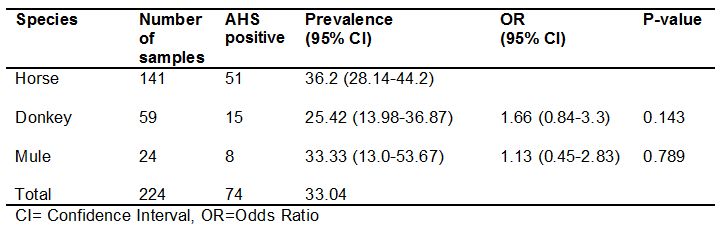
Out of total 224 serum samples collected, 141 samples were from horse, 59 samples were from donkey and 24 samples from mule, with prevalence of 36.2 % (51/141), 25.42% (15/59) and 33.33% (8/24), respectively. Although difference of prevalence appeared between the three species, statistical analysis of result did not show significant difference in the seroprevalence (p>0.05) (Table 4).
Table 4: Seroprevalence of AHS in relation to species.
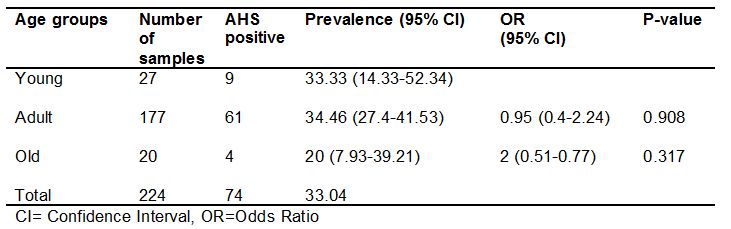
In this study, animals were categorized into three age groups. The first category was young between 4 months to 4 years, the second adult category was 5 - 20 years and the old category was above 20 years with their respective prevalence of 33.33% (9/27), 34.46% (61/177) and 20% (4/20). Like other factors mentioned earlier there was no statistically significant difference between different age groups (p>0.05) (Table 5).
Table 5: Seroprevalence of AHS in relation to the age groups.
5. Discussion
The cross-sectional study conducted on the non-vaccinated equine species (horse, donkey and mule) of the South Western Ethiopia - Damot Gale, Shashogo and Bonke districts - revealed an overall seroprevalence of 33.04% (74/224), indicating the spread of AHS virus throughout the study areas. The presence of AHS antibodies in the sera of non-vaccinated equines was an indication of previous exposure of the equines to nature of infection. The seroprevalence of AHS detected in this study (33.04%) is relatively higher than the study report of Kassa (2006), who reported 23% in three species of equines (horse, donkey and mule) in central Ethiopia. This relative difference could be attributed to difference in geographical location, possibly dictating the vector population. It is important to notice the geographical location where equines reside, which could differ relating to the abundance of midges that are the potential vectors, transmitting the disease among the equine population.
In this study, there was no difference in prevalence as far as sex and age category were concerned and similar finding was reported by Kassa (2006) in the central highlands of Ethiopia. This may be explained by equal exposure rate of both sexes and age categories to the vector. On the other hand, different reports reveal that agroecology of the area where equines reside and species were known to be the potential risk factors for the difference in the prevalence of the disease in the different locations (Kassa, 2006; Barard, 1994). However, this study could not demonstrate such difference. This may be explained either by the mobile nature of the equines in the areas from one agroecology to the other or vector uniformity throughout the physical proximity of the districts. The abundance of midges which are the potential vectors can be predicted from measure of soil moisture content and land surface temperature. Midges breed in damp soils that are rich in organic material, such as irrigated pastures that provide soil moisture adequate for completion of their life cycle (Mellor, 1994). Equines in this study mostly graze in the communal lands, which are swampy, moisten and having temperature suitable for the abundance of equines. This day’s equines (horse, donkey and mule) breed somewhere are brought to other areas for different purposes especially related with cart pulling. This might have also increased their chance to come across different agroecological zones that possibly increase their exposure rate.
6. Conclusions and recommendations
In conclusion, this study has shown the occurrence of AHSV across various agroecological zones of Damot Gale, Shashogo and Bonke Woredas of the South Western Ethiopia. The presence of AHSV, such a devastating virus, poses a serious hindrance to national development, since the affected animals are the means of subsistence for millions in the least privileged parts of the country. Based on the above conclusion, the following points are recommended:
- Awareness should be created to those equine owners to look for timely vaccination.
- There should be further epidemiological study to come up with appropriate control measure.
- There should be development of protective vaccine that incorporates the strain in the area; for this, the virus circulating in the area should be identified and incorporated in the vaccine given.
Annex
Questionnaire format for individual animal
Code_______________ Date_____________
I- Area Information
Region_______________Zone___________Woreda__________PA/Kebele__________
Agroecology: Lowland ____________ Midland ____________ Highland _____________
II- Contact details
Name _______________________________Address ___________________________
I- Animal Information
Species and Breed:
Donkey ________________ Age __________________Sex ______________________
Horse__________________ Age __________________Sex ______________________
Mule___________________ Age __________________Sex ______________________
IV- Modes of Service
Race________________ Pack______________ Cart_______________ Other_______
V- Mobility Pattern
Long distance (>50km) ______________ Medium distance (25-50km) ______________
Short distance (10-25km) ____________
VI- Housing Management
Bran _____________Stable _____________Sharing with people__________________
Stocking Diversity: Alone __________________ With other animals________________
VII- Disease Information
Which species are more affected? (Rank them)
Horse____________________ Donkey___________________ Mule_______________
Mode of Disease Occurrence: Sporadic__ (how many animals were sick in the area) ___
Epidemic____________
Descriptions ___________________________________________________________
VIII- Vaccination Record
______Non-vaccinated ______ Vaccinated, date of last vaccination (dd/mm/yyy) ______
List of abbreviations
AHS - African horse sickness
AHSV - African horse sickness virus
BHK - Baby Hamster Kidney
BP - Blocking percentage
CI - Confidence interval
CL - Confidence level
DsRNA - Double-stranded ribonucleic acid
ELISA - Enzyme-Linked Immunosorbent Assay
Km - Kilometer
MAF - Ministry of Agriculture and Fisheries
ml - Milliliter
mm - Millimeter
MS - Monkey stable
mµ - micro liter
NVI - National Veterinary Institute
OIE - Office of International Epizootics
OD - Optical density
OR - Odds ratio
PBS - Phosphate buffered saline
PCR - Polymerase chain reaction
RNA - Ribonucleic acid
RT-PCR - Real-time polymerase chain reaction
SNNPRS - Southern Nation’s Nationalities and Peoples Regional State
VP - Viral protein
Acknowledgements
Above all, thanks to God, the Almighty Lord of the universe for his innumerable favors and mercy, without which my being and all my efforts of all aspects become meaningless and fruitless.
I am gratitude to all my friends and those persons who unreservedly helped me in all areas of my work.
References
1. Alexander, K.A., Kat, P.W., House, J., House, C., O’Brien, S.J., Laurenson, M.K., MucNutt, J.W., and Osburn, B.I. (1995): African Horse Sickness and African Carnivores. Veterinary microbiology, 47: 133-140.
2. Anon (1998): Foreign animal diseases. United States Animal Health Association, Virginia: Carfor Printing Company, pp. 41-49.
3. Aschalew, Z., Teshale, S., Keith, P. and Feseha, G. (2005):Isolation and Identification of circulating serotype of African Horse Sickness Virus in Ethiopia. The Journal of Applied Research in Veterinary Medicine. Vol. 3, No.1. pp 42.
4. Barard, B.J.H. (1994):Epidemiology of African horse sickness: Zebra virus reservoir. In Foot and mouth Disease, African Horse sickness and pleuropneumonia, summaries and conclusions from the OIE scientific conference, April 1994, OIE, Paris, France.
5. Baylis, M., Mellor, P.S. & Meiswinkel, R. (1999): Horse sickness and ENSO in South Africa. Nature, 397, 574.
6. Binepal, V.S., Wariru, B.N., Davies, F.G., Soi, R. and Olubayo, R. (1992): An attempt to define the host range for African horse sickness virus (Orbivirus, Reoviridae) in East Africa, by a serological survey in some equidae, camalidae, loxo dentidae and carnivore.Veterinary microbiology, 31:19-23.
7. Blen, R., Jovita F., Corinne S., Stephan Z., Sandor B., Marisa, A. and Jose, M.S., (2008): Novel gel-based and real-time PCR assays for the improved detection of African Horse Sickness Virus. Journal of virological methods, in press.
8. Bremer, C.W., Dungu-Kimbenga, B. & Viljeen, G.T. (1998): Detection of African horse sickness virus in Zebra by RT-PCR and the development of different methods for confirming AHSV specificity of RT-PCR products. Proceedings of the Eighth International conference on Equine infectious disease, Dubai, 23-26 March 1998. R and W publications (New market) Ltd, New market, UK.
9. Central statistical authority. (2009): Federal Democratic Republic of Ethiopia. Central Statistical Authority (CSA), agricultural sample silvery 2008/2009 (2001 E.C), Report on livestock characteristics (private peasant holdings), Addis Ababa.
10. Cetre-Sossah, C., Mathieu, B., Setior-Rio, M.L., Grillet, C., Baldet, T., Dellocole, J.C. and Albina, E. (2008): Development and evaluation of a real-time PCR assay for cullicoides imicola, one of the main vectors of bluetongue (BT) and African Horse Sickness (AHS) in Africa and Europe. Research in veterinary science, in press.
11. Coetzer, J.A.W. & Guttrie, A.J. (2004): African horse sickness: In infectious diseases of Livestock, 2nd ed., pp. 1231-1246. Edited by J.A. W. Coetzer & R.C. Tustin. Cape Town: Oxford University press, Southern Africa.
12. Du plessis, D.H., Van Wyncaartd, W., Romito M., Du plessis, M. & Maree, S. (1999):The use of chicken IGY in a double antibody sandwich ELISA for detecting African horse sickness virus.Onderstepoort J. vet. Res., 66, 25-28.
13. European commission (2002):Commission decision of 21 February 2002 amending Annex D to council directive 90/426/EEC with regard to the diagnostic tests for African horse sickness. Off. J. European communities. 153, 37-42.
14. Ferhandey, J., Ferhandey – pechecho, P., Rodriguey, B., Sotelo, E., Robles, A., Arias. M. & Sanchey-vichiano, J.M. (2007):New advances in the molecular diagnosis of African Horse sickness (AHS). Proceedings of the 13th international symposium for the world association of veterinary laboratory Diagnosticians, 11-14 November, Melbourne, Australia.
15. Feseha, G. (1998):Proceedings on equine infectious diseases. 8th international conference on equine infectious disease, Dubai April, pp 318-319.
16. Grubman, M. & Lewis, S. (1992): Identification and characterization of the structural and non-structural proteins of African horse sickness virus and determination of the genome coding assignments. Virology,186, 444-451.
17. Kassa, D, (2006): African horse sickness: seroprevalence and identification of risk factors in equidae at selected sites in Ethiopia. MSc thesis, Addis Ababa University, Faculty of veterinary Medicine, Debre Zeit, Ethiopia, unpublished.
18. Kahn, C.M. and Line, S. (2005): The Merck Veterinary Manual 9thed .White house station, USA. Pp556-557.
19. Koekemoer, M.J.P., Potcieter, A.C., Paweska, J.T. and Van Dijk, A.A. (2000): Development of probe for typing African horse sickness virus isolates using a complete set of clone Vp-2 genes. J. viroll. Methods, 88, 155-144.
20. Maclachlan, N.J., Balasuriya, U.B., Davis, N.L., Collier, M., Johnston, R.E., Ferraro, L.L. and Guthrie, A.J. (2007): Experiences with new generation vaccines against equine viral arteritis, West Nile disease and African horse sickness, vaccine, 25:5577-5502.
21. Maree, S. & pareska, J.T. (2005): preparation of recombinant African horse sickness virus VP1 antigen via a simple method and validation of a VP1 – based indirect ELISA for the detection of group specific IgG antibodies in horse sera. J. virol. Methods, 125 (1), 55-56.
22. Martinez–Torrecaudrada, J., Longeveld, J., Melorn, R. & Caral, I. (2001): Definition of neutralizing sites on African horse sickness virus serotype 4 & VP2 of the level of peptides. J. Gen. Virol., 82, 2415-2424.
23. Meiswinkel, L. and paweska, J.T. (2003): Evidence for a new field Culicoides vector of African horse sickness in South Africa.Preventive veterinary medicine,60:24-53.
24. Mellor, P.S. (1994): Epizootology and vector of African horse sickness virus. Comp. Imm. Microbial. Infect. Dis., vol. 17, No. 3/4: 287-296.
25. Ministry of agriculture and fisheries (1991): surveillance, exotic disease issue, MAF, New Zealand, 18(5), 3-4).
26. Murphy, F.A., Gibbs, E.P.J., Horzinek, M.C., Studdert, M.J. (1999): veterinary virology, 3rd ed. California: Academic press, pp. 400-402.
27. Office International Epizootics (OIE) (2004): African horse sickness, In manual of diagnostic tests and vaccine for terrestrial animals, 5thed, Paris: Office International des Epizootics, pp. 1-21.
28. Office international epizootics (OIE) (2008): African horse sickness, in manual of diagnostic tests and vaccine for terrestrial animals. Paris: Office International des Epizootics, pp. 823-838.
29. Purse, B.V., Mellor, P.S., Rogers, D.J., Samuel, A.R., Merfens, P.P.and Baylis, M. (2005): Climates change and the recent emergence of bluetongue in Europe. Nat Re. Microbial 3, 171-181.
30. Quinn, P.J., Markey, B.K., Carter, M.E., Donnelly, W.J., Leonard, F.C. (2002): Veterinary Microbiology and Microbial Disease. Oxford, England: Blackwell. Scientific; 370
31. Radostitis, O.M., Gay, C.C., Hinchchiff, K.W., and Constable, P.D. (2007): veterinary medicine, A text book of the disease of cattle, sheep, pigs, Goats, and Horse, 10th ed. London: W.B. Saunders company ltd. Pp. 1179-1183.
32. Roy, P., Peter, P., Mertens, C. and Casal, I. (1994): Africa horse sickness virus structure. Comp. Irnrnum. Microbiol. Inject. Dis. Vol. 17, No 3/4: 245-273.
33. Sailleau, C., Hamblia, C., Paweska, J. & Zientara, S. (2000): Identification and differentiation of nine African horse sickness virus serotypes by RT-PCR amplification of the serotype specific genome segment 2 .J.Gen viro., 81, 37-51.
34. Sashi, P., Mahonty, S. and Dutta, K. (1981): Veterinary Virology. Lea and Febiger, Philadelphia, pp172-174.
35. South Nations Nationalities and Peoples State, Agricultural and Rural Development Bureau (2007).
36. Seifert, S.H. (1996): Tropical Animal Health. Wageningen, Germany: Kluwer Academic; 235-240.
37. Sinclair, M. (2006): The epidemiology of African horse sickness outbreak in the Western Cape province of South Africa in 2004. MSc thesis, university of Pretoria.
38. STATA (2006): Stata statistical software, Release 9.0 Texas Stata Corporation, USA.
39. Thrusfield, M. (1995): sampling in veterinary epidemiology, 2nd ed. London: Blackwell science Ltd, PP 179-204.
40. Torrecuadrada, M., Jorge, L., Langeveld, J.P.M., Venteo, A., Sanz, A., Dalsgaard, K., Hamilton, W.D.O., Meloen, R.H. and Casal, J.I. (1999): Antigenic profile of African Horse Sickness virus serotype 4 VP5 and Identification of Neutralizing Epitope shared with Bluetongue virus and epizootic hemorrhagic disease virus. Virology, 257:449-459.
41. Williams, C.F., Inoue, T., Lucus, A.M., Zanatto, P.M.A. and Roy, P. (1998): The complete sequence of four major structural proteins of African horse sickness virus serotype 6: evolutionary relationship within and between the Orbiviruses. Virus research,53: 55-73.
Cite this paper
APA
Demissie, G. H. (2013). Seroepidemiological Study of African Horse Sickness in Southern Ethiopia. Open Science Repository Veterinary Medicine, Online(open-access), e70081919. doi:10.7392/Research.70081919
MLA
Demissie, Gizachew Hailegebreal. “Seroepidemiological Study of African Horse Sickness in Southern Ethiopia.” Open Science Repository Veterinary Medicine Online.open-access (2013): e70081919.
Chicago
Demissie, Gizachew Hailegebreal. “Seroepidemiological Study of African Horse Sickness in Southern Ethiopia.” Open Science Repository Veterinary Medicine Online, no. open-access (January 21, 2013): e70081919. http://www.open-science-repository.com/seroepidemiological-study-of-african-horse-sickness-in-southern-ethiopia.html.
Harvard
Demissie, G.H., 2013. Seroepidemiological Study of African Horse Sickness in Southern Ethiopia. Open Science Repository Veterinary Medicine, Online(open-access), p.e70081919. Available at: http://www.open-science-repository.com/seroepidemiological-study-of-african-horse-sickness-in-southern-ethiopia.html.
Nature
1. Gebreal, G. H. Seroepidemiological Study of African Horse Sickness in Southern Ethiopia. Open Science Repository Veterinary Medicine Online, e70081919 (2013).
Science
1. G. H. Demissie, Seroepidemiological Study of African Horse Sickness in Southern Ethiopia, Open Science Repository Veterinary Medicine Online, e70081919 (2013).
doi
Research registered in the DOI resolution system as: 10.7392/Research.70081919.

This work is licensed under a Creative Commons Attribution 3.0 Unported License.