Open Science Repository Engineering
doi: 10.7392/Engineering.70081906
Transesterification and Optimization of Groundnut Oil Using Magnetic Stirrer
Department of Chemical Engineering, Federal University of Technology, P.M.B 1526, Owerri, Nigeria
Abstract
The alkali-catalyzed transesterification of groundnut oil using a magnetic stirring device was carried out to determine the optimal values of process variables that would result in the maximum yield of biodiesel. The free fatty acid content (FFA) of the oil was determined to be 1.86%; hence, this justified the choice of the alkali-catalyzed process route. The process variables involved are alcohol-to-oil molar ratio, catalyst concentration, reaction temperature and time. The optimal conditions for the alkali-catalyzed transesterification were found to be an alcohol-to-oil ratio of 5:1, catalyst concentration of 0.5%w/w, a reaction temperature of 70oC and 50 minutes reaction time. The biodiesel yield at these optimal conditions obtained in this work was 87.1%. The biodiesel was found to have a flash point of 167oC, a cloud point of 8oC, pour point of -8oC and kinematic viscosity and specific gravity of 8 and 0.84, respectively. The properties of the biodiesel were compared to the ASTM standard for diesel.
Keywords: biodiesel, transesterification, characterization, yield, optimization, reaction condition, groundnut oil.
Citation: Oghome, P. I. (2012). Transesterification and Optimization of Groundnut Oil Using Magnetic Stirrer. Open Science Repository Engineering, Online(open-access), e70081906. doi:10.7392/Engineering.70081906
Published: November 23, 2012
Copyright: © 2012 Oghome, P. I. Creative Commons Attribution 3.0 Unported License.
Email: [email protected]
Introduction
As civilization grows, the transportation or movement of persons and goods plays a crucial role in daily living. One of the greatest challenges facing man is the growing population in face of fossil fuel depletion. Over hundred years, the major source of energy shifted from solar to fossil fuels, i.e. hydrocarbons and coal. As technology improves, the use of fossil fuels increases as well, thus this leads to the necessity of searching for alternative sources of energy. Biodiesel is an alternative fuel made from renewable sources, such as vegetable oils (edible and non- edible oils) animal fats. It can be used as an alternative fuel for diesel engines (Berchmans et al, 2008), and biodiesel has also drawn considerable attention during the past decade as a renewable, biodegradable and non- toxic fuel.
Essentially, biodiesel is a mono alkyl esters of fatty acid derived from vegetable sources and animal fat. (Xin et al, 2010). It has similar and sometimes better physical and chemical properties than petroleum diesel, such as higher flash point, ultra-low sulphur concentrations, better lubricating efficiency and little pollutant production. It has an energy content of about 12% less than petroleum diesel fuel on a mass basis (Rahman et al, 2010). Recently, there has been increasing concern towards the welfare of our environment and atmosphere. The major factors that degrade the environment have been identified with emissions from the use of fossil fuels. Furthermore, due to the predicted limited existence of conventional fuels, alternative fuel sources must be created to check the problems presented above. Biofuels have proven to be environmental friendly and possessing favourable combustion profiles. Consequently to these facts, the importance of this research cannot be ignored, since the study of the feasibility of biodiesel production contributes to the advancement in the development of biofuels and its impact on humans.
Biodiesel is a light to dark, pale yellow liquid, it is practically immiscible with water, has a high boiling point and low vapor pressure. It is produced by the transesterification of fats and oils with alcohols in the presence of an acid or alkaline catalyst, with an impure co-product, glycerol (Yusuf et al, 2009). Biodiesel commonly referred to as fatty acid methyl esters (FAME) can be produced from renewable sources.
Animal fats and plant oils are composed of triglycerides which are esters of three free fatty acids. In transesterification process, alcohol is deprotonated with a base to make it a stronger nucleophile. Commonly, ethanol or methanol is used. In general, a large excess methanol is used to shift the equilibrium far to the right. Transesterification reactions can be alkali-catalyzed, acid-catalyzed or enzyme-catalyzed (Zhang et al, 2003). The overall reaction of transesterification is shown in equation 1:

The first two types of catalyzed reactions have received the greatest attention and are currently used today. The same cannot be said for the enzyme-catalyzed reaction, because it requires a much longer reaction time than the other two systems. It has been established that transesterification is dependent on several major process variables. These include: molar ratio of alcohol to oil, catalyst concentration, reaction temperature and time .Molar ratio of alcohol to oil plays a vital role in biodiesel yield. From stoichiometry, the transesterification reaction requires three moles of alcohol per mole of triglyceride but, due to the reversible nature of the reaction, excess alcohol increases the conversion of oils into methyl esters within a short time, and this translates to the fact that biodiesel yield increases with the increase in the amount of alcohol used in the reaction up to a certain concentration (Mathiyazhagan and Ganapathi, 2011). However, further increase in the amount of alcohol does not increase the yield of biodiesel; instead, increases the cost of alcohol recovery (Leung and Guo, 2006). Furthermore, alcohol content may vary with catalyst used. For oil samples with high FFA, transesterification does not respond to alkali catalyst.
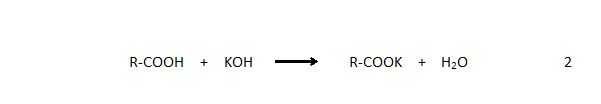
The water and free fatty acid (FFA) contents of groundnut oils are critical factors that inhibit transesterification reaction (Mathiyazhagan and Ganapathi, 2011). Free fatty acids are carboxylic acids of large alkyl groups present in triglycerides, oils and fats in varying degrees. If oil samples have high FFA content (>1%), then the transesterification requires more alkali catalyst to actually neutralize the free fatty acids present in the oil sample. This is represented in the equation 2:
A high amount of FFA and water in oil samples deactivates catalysts,
and addition of excess amount of catalyst, as compensation for the
deactivating effect of the FFA, gives rise to the formation of
emulsion and gels, i.e. soap, which results in viscosity increase
and problems associated with glycerin separation and loss in yield of
methyl esters (Berchmans et al, 2008).
Biodiesel production is also affected by the concentration of catalyst used.
Most commonly used catalysts for biodiesel production are sodium and
potassium hydroxide. Generally, conversion of triglycerides into
biodiesel increases with increasing catalyst concentration. However,
at some point, the optimal catalyst concentration is reached (when
other variables are fixed) and further increase in catalyst
concentration would have negative impact on product yield, because
excess amount of alkali catalyst reacts with the triglycerides
present in the oil to form soap (Leung and Guo, 2006). Berchmans et
al (2008) investigated the alkaline transesterification of crude palm
oil and coconut oil by changing catalyst NaOH to oil ratios (%,w/w)
and catalyst to oil ratios (%,w/w). High conversion was obtained with
1.0% of catalyst NaOH-to-oil ratio and 28% of methanol to oil ratio,
and under these conditions the FAME yield was 80 % for crude palm oil
and 85 % for coconut oil. Freedman et al (1986) observed the increase
in fatty acid ester conversion when there is increase in reaction
time. The reaction tends to proceed slowly at the beginning, due to
mixing and dispersion of alcohol and oil, but with time the reaction
proceeds fast. Further increase in reaction time (above the optimal
time) does not increase yield rather, longer reaction time leads to
reduction in yield due to the reversible nature of
transesterification, resulting in loss of esters as well as soap
formation (Leung and Guo, 2006).
Reaction temperature is another essential factor in biodiesel production.
Higher reaction temperature translates to increased rate and shortens
the reaction time due to the reduction in viscosity of the oil. The
reduction in oil viscosity enhances mass transfer between the
reactants and promotes intimate contacting. However, temperature
increase beyond optimal levels leads to decrease in biodiesel yield
because, at higher reaction temperatures, the saponification of
triglycerides is accelerated. In addition, transesterification
temperature should be below the boiling point of the alcohol used to
prevent alcohol evaporation. The range of optimal reaction
temperature depends upon the oil or fat used (Mathiyazhagan and
Ganapathi, 2011).
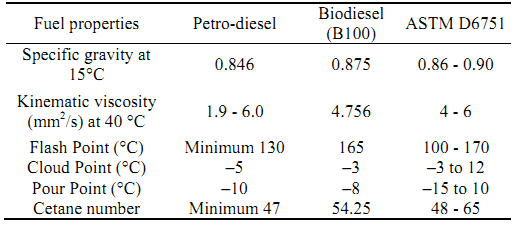
The fuel properties of biodiesel are cloud point and pour point, flash
point, viscosity and cetane number. Cold temperature behaviour of
biodiesel is an important quality criterion, as frozen fuel may cause
blockage of the fuel lines and filters and starve the engine of fuel.
The two most important criteria are the cloud and pour points. Cloud
point is the temperature at which wax first becomes visible when the
fuel is cooled while (Bello et al., 2011). Pour point is the temperature at which the
amount of wax out of solution is sufficient to gel the fuel, thus it
is the lowest temperature at which the fuel can flow. The cold point
characteristics of biodiesel products depend on chain length and
degrees of unsaturation, with long chain saturated fatty acid esters
displaying particularly unfavourable cold temperature behaviour
(Rahman et al., 2010). The cloud, pour and cold filter plugging points
are above zero, which limits their application in cold regions.
Flash point is the lowest temperature to a barometric pressure of 101.3
kPa, at which a liquid sample produces sufficient vapour for the
air-vapour mixture above the surface to flash momentarily on exposure
to a standard source of ignition. It is a measure of flammability of
fuels and, thus, an important safety criterion in transport and
storage. The flash point of pure biodiesels is usually higher than
the ASTM limits, but fall rapidly with increasing amount of methanol.
Ground nut oil (GNO) has a flash point of 165oC and the
value for FAME is 141oC which, although are below the
minimum for biodiesel, are higher than that for diesel fuel (Suleiman
et al, 2012).
Kinematic velocity is the ratio of the dynamic viscosity of a substance to its
relative density. The kinematic viscosities of the GNO and FAME are
244.52 and 6.6 mm2/s respectively. Viscosity is an
important property of biodiesel since it affects the operation of
fuel injection equipment, particularly at low temperatures, when the
increase in viscosity affects the fluidity of the fuel, or leakage at
high temperature when too thin.
Cetane number (CN) is a measure of the ignition quality of diesel fuels and
one of the prime indicators of the quality of diesel fuel. It relates
to the ignition delay time of a fuel upon injection into the
combustion chamber; it influences ease of starting, duration of white
smoke after start up, drivability before warm up and intensity of
diesel knock at idle. Biodiesel has a higher cetane number than
diesel fuel because of its oxygen content and also because the fatty
acids present in the fuel have very high octane number. Cetane number
increases with increasing length of both fatty acid chain and ester
groups, while it is inversely related to the number of double bonds.
It has been shown that the cetane number of biodiesel depends
essentially on the distribution of fatty acids in the feedstock
(Gerpen, 2005).The longer the fatty acid carbon chains and the more
saturated the molecules, the higher the cetane number. The calculated
cetane number of the GNO of 26 increased to 51 for the fatty acid
methyl esters.
Table 1: Comparison of fuel properties of petro-diesel, bio-diesel and ASTM D6751.
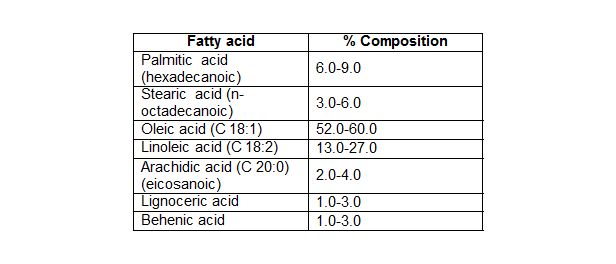
Groundnut (peanut) oil is classified, among others, as oleic-linoleic acid oil, seeing that they contain a relatively high proportion of unsaturated fatty acids, such as the monounsaturated oleic acid and the polyunsaturated linoleic acid. They are characterized by a high ratio of polyunsaturated fatty acids to saturated fatty acids. As a consequence of this, they have relatively low melting points and are liquid at room temperature (Suleiman et al., 2012).
Table 2: Fatty acid composition of groundnut oil.
Source: Chempro.
Experimentation
Reagents and materials
The experiment was carried out at the Ministry of petroleum and environment laboratory Owerri, Imo State. Groundnut oil was purchased in Ihiagwa in Imo State. Methanol of certified purity of 99 % and Sodium hydroxide of analytical grade was used.
Transesterification procedure
The free fatty acid test was first performed to determine the percentual FFA content of the oil sample, and a 1.86% FFA content resulted from the test. This justified the choice of alkaline transesterification route. Process variables were varied to investigate their influence on methyl ester yield. They include catalyst NaOH-to-oil concentrations (0.5% w/w, 1.0% w/w, 1.5% w/w and 2.0% w/w), methanol-to-oil ratios (4:1, 5:1, 6:1, 9:1), temperature (50oC, 60oC, 70oC, 80oC and 90oC) and reaction time (20 min, 30 min, 40 min, 50 min, 60 min and 90 min). 50 grams of oil was used throughout the experiment. All reactions were carried out in conical flasks placed on a magnetic stirrer. Each process variable was varied while others were kept constant. After the reaction, the mixture was allowed to settle for 5 h to overnight, before separating the glycerol layer from the top layer, i.e. methyl ester fraction. The separated methyl ester fraction was washed with warm distilled water in a separating funnel and dried in an oven. The final methyl ester yield was weighed and recorded.
Results and discussion
The experimental results are shown in Tables 3 to 7 and are represented in Fig. 1 to 4, respectively.
Table 3: Effect of molar ratio on yield.
Mass of oil 50 g, temperature of 70°C, time 20 min, 0.5% w/w catalyst amount.
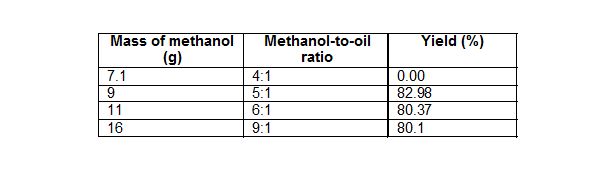
Fig. 1. Effect of mole ratio of methanol to oil on biodiesel yield at constant temperature of 70°C, time 20 min, and catalyst concentration 0.5 % w/w.
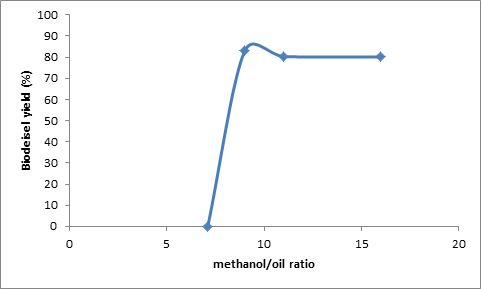
Table 4: Effect of catalyst concentration on yield.
Mass of oil 50 g, temperature of 70°C, time 20 min, molar ratio of 5:1.
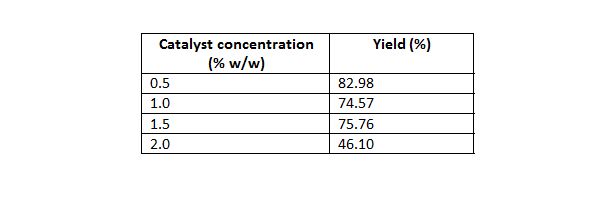
Fig. 2. Effect of catalyst concentration on biodiesel yield at constant temperature of 70 °C, time 20 min, and mole ratio 5:1.
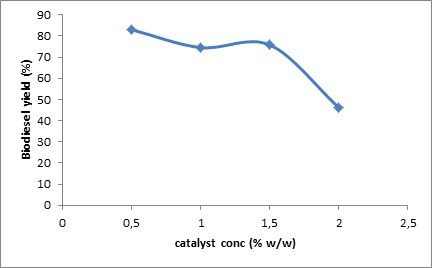
Table 5: Effect of reaction temperature on yield.
Mass of oil 50 g, time 20 min, molar ratio of 5:1, 0.5 % w/w catalyst amount.
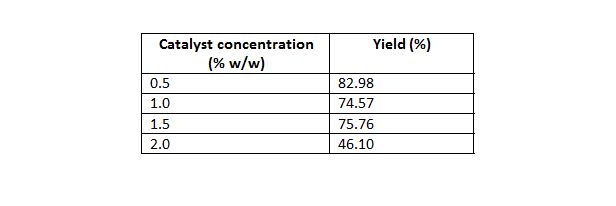
Fig. 3: Effect of temperature on biodiesel yield at constant time of 20 min, molar ratio 5:1, and catalyst concentration of 0.5 % w/w.
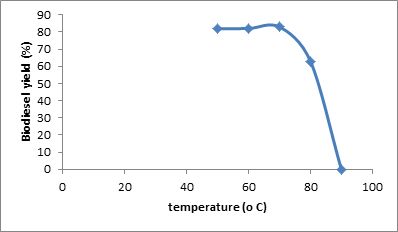
Table 6: Effect of reaction time on yield.
Mass of oil 50 g, temperature 70°C, molar ratio of 5:1, 0.5 % w/w catalyst amount.
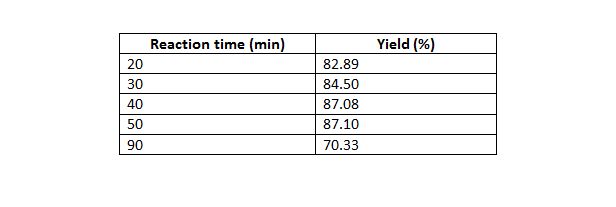
Fig. 4: Effect of reaction time on biodiesel yield at constant mole ratio 5:1, catalyst concentration of 0.5 % w/w and temperature of 70 °C.
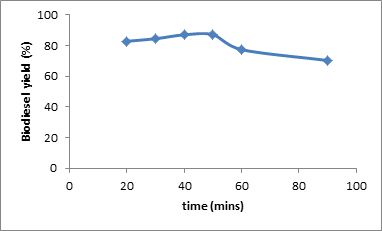
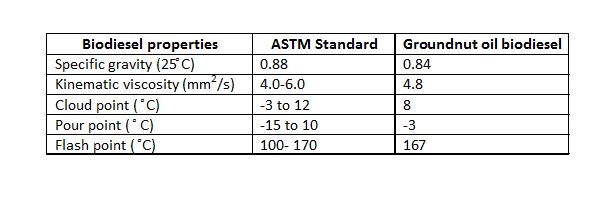
Table 7: Properties of groundnut biodiesel.
Stoichiometrically, the methanolysis of vegetable oil requires three moles of methanol for each mole of oil. Since transesterification of triglycerides is a reversible reaction, excess methanol is required to shift the equilibrium towards the direction of ester formation. From Fig. 1 at 70C, the maximum yield was achieved at methanol to oil molar ratio of 5:1. As the mote ratio was increase to 6:1 and 9:1, there was a drop in yield. Hanh et al. (2007) obtained 90% conversion using methanol as an alcohol with triolein oil to alcohol molar ratio of 1:6 and KOH as a catalyst with ultrasonic irradiation. Furthermore, Kumar et al. (2010) have obtained above 98% yield using 1:9 Jatropha oil to methanol molar ratio and heterogeneous solid catalyst used was NaSiO2. No methyl ester yield was achieved using a 4:1 molar ratio, and this can be attributed to the predominance of esterification reaction at the initial phase of transesterification to transesterify the FFA present in the Jatropha oil, which can consume methanol present in the reaction mixture, and, hence, the amount of methanol available for transesterification may not be sufficient to drive the reaction forward for longer time.
Effect of variation of amount of catalyst on conversion was also studied. Catalyst amount was varied in the range of 0.5% to 2.0% (w/w of the oil taken). As shown in Fig. 2, methyl ester yield decreased steadily with increase in catalyst amount due to soap formation. Kumar et al. (2010) obtained their best result at 3 % w/w of solid NaSiO2 catalyst amount, which is higher than the present study. Hanh et al. (2007) obtained about 90% conversion with 1% w/w of NaOH catalyst.
The result of this study showed that a maximum yield of methyl ester was achieved at temperature of 70oC. However, no separation was obtained after the reaction was carried out at 90oC and a homogeneous mixture was obtained. This can be attributed to the presence of soap molecules, as saponification of triglycerides is favored at high temperatures. The presence of soap molecules in biodiesel causes difficulties in separation as emulsions and gels are formed. The influence of temperature on yield is shown in Fig. 3 The effect of time on methyl ester yield was also studied. Reaction time was varied at 20, 30, 40,50, 60 and 90 minutes. The result indicates a maximum biodiesel yield of 87.1% at a reaction time of 50 minutes. Fig. 4 describes the effect of reaction time on methyl ester yield.
Conclusion
From the results obtained, alkali-based transesterification reaction for the production of biodiesel was found to be very promising. The optimum conditions for the production of biodiesel from groundnut oil were a molar ratio of 5:1, 0.5% w/w catalyst concentration at 70oC for a duration of 50 minutes. On characterization and comparison, the biodiesel produced was found to conform to the ASTM D6571 biodiesel standard, with a specific gravity of 0.84, kinematic viscosity of 4.8, cloud point of 8oC, flash point of 167oC, which make it a safe fuel for handling and storage. The transesterification of groundnut oil provides a possibility for producing cheap alternative fuels, which will reduce pollution and protect the environment.
References
1. Berchmans, H. J., & Hirata, S. (2008). Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Bioresource Technology, 99(6), 1716-1721. FIND ONLINE
2. Freedman, B., Butterfield, R. O., & Pryde, E. H. (1986). Transesterification kinetics of soybean oil. Journal of the American Oil Chemists' Society, 63(10), 1375. FIND ONLINE
3. Van Gerpen, J. (2005). Biodiesel production and fuel quality. Department of Biological and Agricultural Engineering, University of Idaho, Moscow, ID. FIND ONLINE
4. Hanh, H. D. (2007). Effects of molar ratio, catalyst concentration and temperature on transesterification of triolein with ethanol under ultrasonic irradiation. Journal of the Japan Petroleum Institute, 50(4), 195-199. FIND ONLINE
5. Kumar, D., Kumar, G., & Singh, C. P. (2010). Ultrasonic-assisted transesterification of Jatropha curcus oil using solid catalyst, Na/SiO₂. Ultrasonics Sonochemistry, 17(5), 839-844. FIND ONLINE
6. Leung, D. Y. C., & Guo, Y. (2006). Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Processing Technology, 87(10), 883-890. FIND ONLINE
7. Mathiyazhagan, M., & Ganapathi, A. (2011). Factors affecting biodiesel production. Research in Plant Biology, 1(2), 1-5. FIND ONLINE
8. Rahman, K. M., Mashud, M., Roknuzzaman, M., Al Galib, A., Uddin, M. N., Mashud, M., ... & Abdullah-Al-Nahian, S. M. S. (2010). Biodiesel from Jatropha oil as an alternative fuel for diesel engine. International Journal of Mechanical & Mechatronics (IJMME-IJENS), 10(3), 1-6. FIND ONLINE
9. Suleiman M. M., Bello A.U., Itumoh J. E., Bello K ., Bello A. M., and Arzika, A. T. (2012). Physicochemical properties of some commercial groundnut oil products sold in sokoto metropolis, Northwest Nigeria. Journal of Biological Science and Bioconservation, 4. FIND ONLINE
10. Deng, X., Fang, Z., Liu, Y. H., & Yu, C. L. (2011). Production of biodiesel from Jatropha oil catalyzed by nanosized solid basic catalyst. Energy, 36(77), 7e784. FIND ONLINE
11. Yusuf, N., Salaudeen, N., & Muhammad, F. (2011). An Experimental Study of Biodiesel Synthesis Using Groundnut Oil. Part II: Kinetics Study of the Reaction. Australian Journal of Basic and Applied Sciences, 5(6), 912-919. FIND ONLINE
12. Zhang, Y. A., Dube, M. A., McLean, D., & Kates, M. (2003). Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresource technology, 89(1), 1-16. FIND ONLINE
13. Bello, I. E., Mogaji, T. S.,& Agge, M.(2011). The effects of transesterification on selected fuel properties of three vegetable oils. Journal of Mechanical Engineering Research, 3(7), pp. 218–225. FIND ONLINE
14. Strong, C, Erickson, C & Deepak S (2004). Evaluation of biodiesel fuels: literature review. Monograph. College of Engineering, Montana State University-Bozeman. FIND ONLINE
Cite this paper
APA
Oghome, P. I. (2012). Transesterification and Optimization of Groundnut Oil Using Magnetic Stirrer. Open Science Repository Engineering, Online(open-access), e70081906. doi:10.7392/Engineering.70081906
MLA
Oghome, P. I. “Transesterification and Optimization of Groundnut Oil Using Magnetic Stirrer.” Open Science Repository Engineering Online.open-access (2012): e70081906. Web. 23 Nov. 2012.
Chicago
Oghome, P. I. “Transesterification and Optimization of Groundnut Oil Using Magnetic Stirrer.” Open Science Repository Engineering Online, no. open-access (November 23, 2012): e70081906. http://www.open-science-repository.com/transesterification-and-optimization-of-groundnut-oil-using-magnetic-stirrer.html.
Harvard
Oghome, P.I., 2012. Transesterification and Optimization of Groundnut Oil Using Magnetic Stirrer. Open Science Repository Engineering, Online(open-access), p.e70081906. Available at: http://www.open-science-repository.com/transesterification-and-optimization-of-groundnut-oil-using-magnetic-stirrer.html
Science
1. P. I. Oghome, Transesterification and Optimization of Groundnut Oil Using Magnetic Stirrer, Open Science Repository Engineering Online, e70081906 (2012).
Nature
1. Oghome, P. I. Transesterification and Optimization of Groundnut Oil Using Magnetic Stirrer. Open Science Repository Engineering Online, e70081906 (2012).
doi
DOI name: 10.7392/Engineering.70081906

This work is licensed under a Creative Commons Attribution 3.0 Unported License.