Open Science Repository Chemistry
doi: 10.7392/Chemistry.70081936
Behavior of Chromate Ions in CaO-Al₂(SO₄)₃ Suspension
Chemistry Department, Community College for Girls in Hyanakiyah, Taibah University, Al-Madinah Al-Munawarah, Saudi Arabia
Abstract
Behavior of the chromate ions and their removal efficiency in CaO-Al₂(SO₄)₃ suspensions with molar ratio 5:1 was studied. The suspension was prepared by agitating with a magnetic stirrer for constant 3 hours at room temperature. Run products were collected by filtration, washing and air-dry and evaluated by XRD, SEM and DTA. According to XRD and SEM results the major product in this suspension was ettringite with minor amounts of gypsum and calcite. DTA results showed that the presence of the chromate ions in the suspension retards the formation of the products and decreases the degree of their crystallinity. However, there is high removal of the chromate ions by the formed suspension products. This is related to the substitution of the chromate ions by sulfate in the ettringite crystals.
Keywords: CaO-Al₂(SO₄)₃, suspensions, chromate ions, removal.
Citation: AbuAlola, K. A. A. (2013). Behavior of Chromate Ions in CaO-Al₂(SO₄)₃ Suspension. Open Science Repository Chemistry, Online(open-access), e70081936. doi:10.7392/Chemistry.70081936
Received: February 2, 2013
Published: March 6, 2013
Copyright: © 2013 AbuAlola, K. A. A. Creative Commons Attribution 3.0 Unported License.
Contact: [email protected]
1. Introduction
The presence of heavy metals in the environment has been of great concern because of their growing discharge, toxicity and other adverse effects on receiving waters. Heavy metals contaminants in soils originate in the spreading of inorganic fertilizers, sewage sludge and industrial wastes. One of those toxic species is chromium and its chromate derivatives, which, if present in levels greater than the permissible limit, become long-term hazardous contaminants because of their high toxicity, causing severe damage to the kidneys and nervous system. In alkaline solutions, Cr (VI) primarily occurs as CrO42- and Cr2O72-(1). CrO42- and Cr2O72- ions are somewhat soluble and can escape into aqueous leaching solutions. The problems of determination and reduction of soluble chromates are of great interest, and this can be verified looking at the several technical papers and patents that have been presented on this topic during the last years (2, 3).
Suspensions of saturated solutions of calcium oxide with aluminium sulfate will result in different products and depend on many factor like the molar ratio of calcium oxide to aluminium sulfate, initial pH of the solution and concentration of sulfate ions (4)in the suspensions. If the calcium oxide:aluminium sulfate molar ratio is more than 4, a product called ettringite, (3CaO.Al2O3. 3CaSO4. 32H2O), is formed. Ettringite is a naturally occurring mineral found in Germany for the first time (5,6). This mineral is characterized by the very high content of water molecules and is very important to the cement technology, since it appears as an early hydration product for the first stage of hydration of Portland cement. Recently, ettringite attracted a special attention in view of environmental issues, specifically in sub-surface geology concerning SO3-comprising waste dumping and fluorine sorption from contaminated waste waters as well as underground waters (7-9). Chromate ions which may present as a contaminate in the waste water can substitute sulfate ions in the crystal structure of ettringite and, for this, ettringite represents a good reagent for their removal(10-12).
In the present study, removal behavior of chromate ions by CaO-Al2(SO4)3 suspension is studied. The characteristics of the products after various time intervals were studied using X-ray diffraction (XRD), scanning electron microscope (SEM) and differential thermal analysis (DTA).
2. Methodology
2.1. Preparation of the suspensions
Reagents of Ca oxide, CaO, special grade of Sigma Aldrich, BET specific surface area 13.37 m2/g, and Al-sulfate hydrate, Al2(SO4)3 .(14-18)H2O were used. Primarily, Al-sulfate solution was prepared by diluting with deionized water to obtain 0.01mol/L solution as Al2(SO4)3 which mixed with 100 ml water containing 0.05 mol/L CaO in presence of various concentrations of chromate ions. Different mixes were prepared designated as E0, E1, E2, E3, E4 and E5 with concentrations of CrO42- ions 0, 0.01, 0.02, 0.03 and 0.05 molar/l respectively. Each suspension was agitating by a magnetic stirrer for constant 3 hours at room temperature. After 1, 2, 4, 6 and 24 hours of mixing process, filtration was carried out and the filtrate was stored for determination of the remains of CrO42- ions. The precipitation remained in the filter paper was washed out by distilled water and dried in air for 24 hours. After drying, it was stored in a desiccator containing silica gel for analysis. The precipitated was evaluated by XRD, SEM and DTA.
2.2 Evaluation of the products
The products produced from the calcium oxide, aluminum sulfate suspension were evaluated by using X-ray diffraction analysis (XRD), scanning electron microscope (SEM) and differential thermal analysis (DTA). X-ray examination was carried out by employing Mac Science MXP3 diffractometer under 40kV-20mA CuKα radiation. The growth of the products crystals was studied by SEM examination. A JEOL-JSM-5400 high resolution scanning electron microscopy was used (Shimadzu Co., Japan). For DTA test, differential thermal analyzer was used at heating rate of 20°C/min. The measurements were made in N2 atmosphere using Shimadzu DTA - 50H. The sample chamber was purged with nitrogen at a flow of 30 ml/min.
2.3. Behavior of the removal of chromate ions
The remains of CrO42- ions in the filtrate were measured by colorimetric techniques. This was done using colorimeter at 370 nm, corresponding to the maximum absorbency of CrO42- ions. The uptake percentage was calculated using the following equation:
Removal (molar/l) = [(C0 - Ct) ]
Where: C0 is the initial concentration of CrO42- ions insert in suspensions (molar/l) and Ct is the remained concentration of CrO42- ions in the filtrate after various runs (molar/l).
3. Results and discussion
3.1 X-ray diffraction
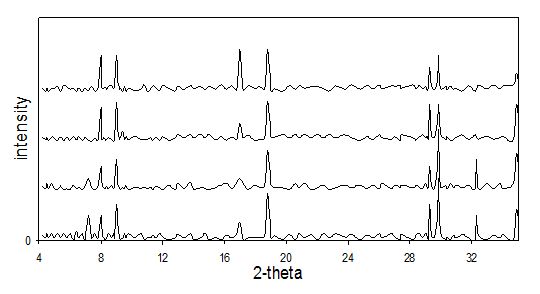
XRD patterns of E0, E1, E3 and E4 mixes after 6 hours of mixing are shown in Fig.1. For mix E0, the main product was ettringite with minor amounts of the gypsum (CaSO4), gibbsite (Al(OH)3) phases. This is indicated by the increase in the intensity of the peaks characterized to ettringite compared to those of the other products. Calcite, CaCO3, was also identified in X-ray patterns. Minor calcite formation was also reported by some authors during synthesis of ettringite in suspension solutions (8, 13, 14, 15).Peaks of unreacted CaO and Al2(SO4) were also identified in E0 patterns. For E1, E3 and E4 suspensions, similar products were obtained but with slight lower intensities in the peaks characterized to the obtained products (ettringite, gypsum and calcite), and there is an increase in the intensities of the peaks of the reactants. This decrease in the intensities of the products peaks accompanied with increase in the intensities of reactants peaks indicates a retardation to the reaction between CaO and Al2(SO4) as a result of presence of chromate ions. This retardation to the main reaction is due to side reaction, which is the substitution of the chromate ions by the sulfate ions inside the ettringite crystal.
After 24 hours of mixing process, XRD patterns of the different mixes were represented in Fig. 2. From the obtained patterns of E0 suspension, we can notice that the intensity of the ettringite and the other reaction products were increased while the intensity of CaO and Al2(SO4) decreased, indicating progress of the reaction. Besides there are increased intensities of the gypsum phase in the suspensions containing chromate ions, comparing to E0 suspension. This indicates an increase in its formation in these suspensions. Here, there is no significant difference between the intensities of the peaks identified for the reaction products (gypsum and ettringite) for E0 and the other mixes.
It is obvious that ettringite formation and its amounts in the suspension, comparing to other products, depend on molar ratio of calcium oxide to aluminium sulfate in the starting suspensions. Also, their precipitation depends on pH of the solutions (10) which is also a function of this molar ratio in suspensions. Strictly saying, ettringite precipitation may depend on the concentrations of ionic species dissolved in the suspensions. Here, although the ratio of calcium oxide to aluminum sulfate used in the suspension was 5:1, not all CaO is soluble. This permits the formation of other products beside ettringite, like gypsum and calcite. However, the main reaction product is ettringite, which explains the high uptake of the chromate ions noticed in the later section.
3.2. Scanning electron microscope
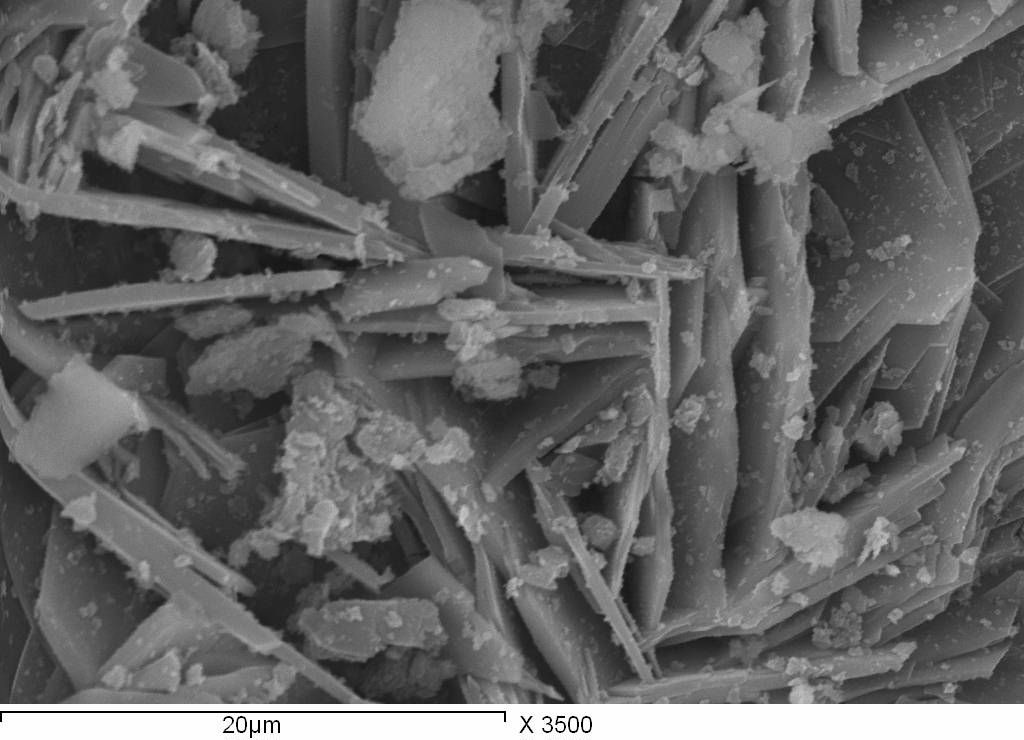
SEM micrographs of the precipitate formed from E0 and E3 mixes after 6 hours of mixing are shown in figures 3 and 4 respectively.
Fig. 3 represents SEM micrograph for E0 mix, after 6 and 24 hours of mixing process. The micrographs showed a well definite crystal which can be related to ettringite beside to platy crystals that can be related to CaO. This confirms that the main products of the reaction of CaO and Al2(SO4) suspensions is the etringite. After 24 hours of mixing the suspensions, Fig.3- b, there is an increase in the size and the crystallinity of the formed ettringite.
For E3 suspension after 6 hours, Fig 4 - a, smaller ettringite crystals with a fewer numbers can be identified in the micrographs after 6 hours of preparing the suspensions. This confirms that the presence of chromate ions retards the rate of formation of ettringite. After 24 hours, higher numbers of ettringite crystal can be observed in SEM micrographs, Fig.4 - b. The reduction in the ettringite crystal noticed here in the micrographs agrees with the decrease in the intensities of XRD peaks obtained at the same mixing age and has the same explanation.
3.3. Differential thermal analysis
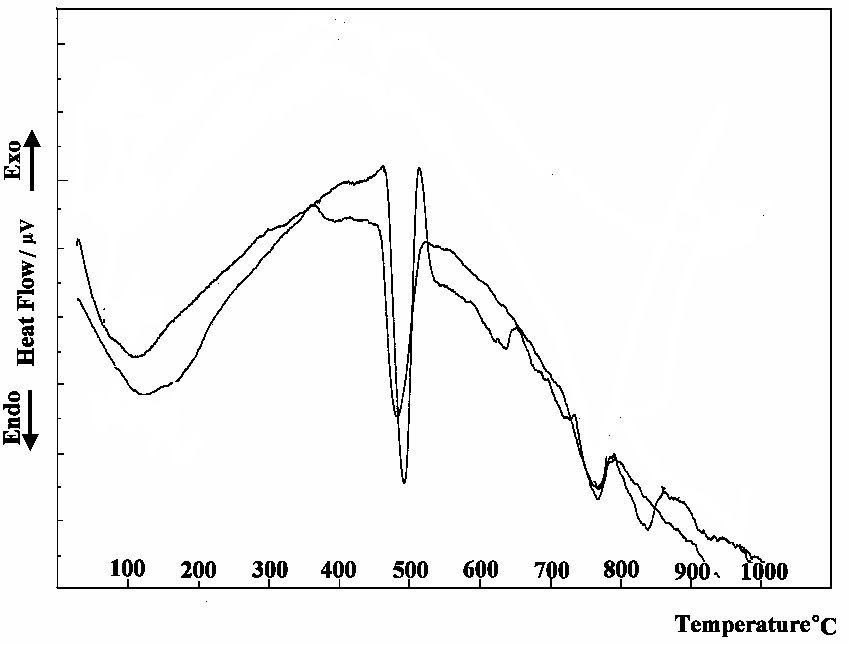
The DTA thermogrames of precipitates produced from suspensions E0 and E3 after 6 hours of mixing are shown in Fig. 5. All DSC curves showed three main endothermic peaks located at 105, 490, and 700-780 ºC. The first endotherm located at 105 ºC is mainly due to the dehydration of the ettringite, which is the main suspension product. The second peak located at 490 ºC, represent the major mass loss, mainly related to the decomposition of CaO (16). We can noticed the increase in the intensity of such peak in case of suspension E3, which confirms the retardation effect to the reaction of CaO and Al2(SO4)3 as a results of the presence of chromate ions.
The third endothermic double peak located at 718-770 ºC is due to the decomposition of calcite, CaCO3 with different degrees of crystallinity (17,18). The enthalpy of this endotherm varies as a result of change in the degree of carbonation of the specimens.
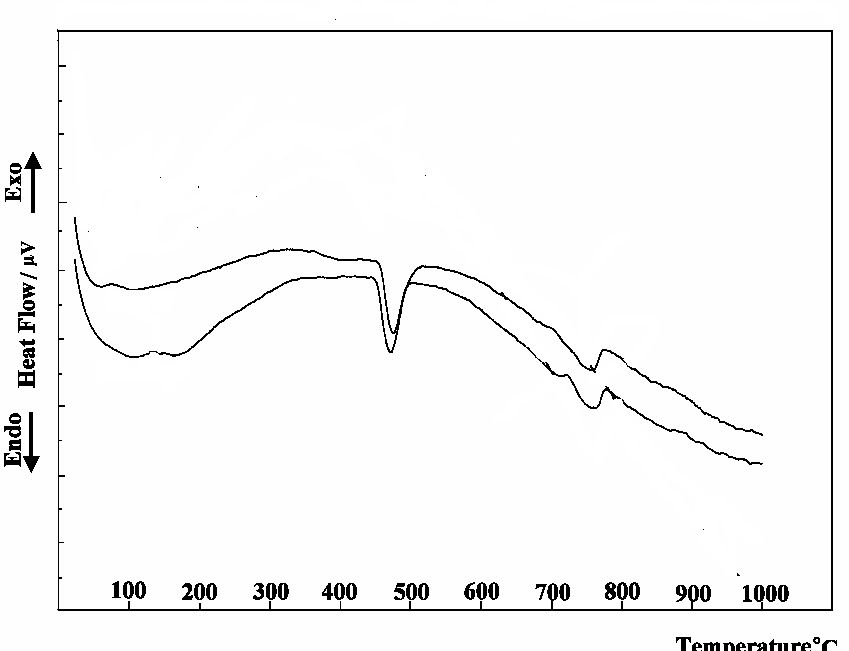
After 24 hours of mixing, the SDC curves for E0 and E3 are shown in Fig. 6. The same three endothermic peaks are observed as in the case after 6 hours of mixing. While we can notice the decrease in the intensity of the second peak characterized by CaO with an notable increase in the intensity of the first peak characterized by ettringite, the major reaction products. From that, we can concluded the progress of the reaction.
3.4. Removal behavior of CrO4-2 ions
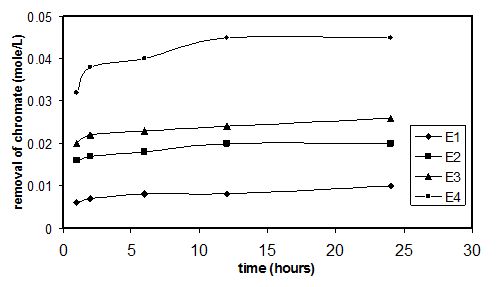
The rate of removal of CrO42- ions (mole/l) by CaO- Al2(SO4) a function of the mixing time was shown in Fig.(7).
The amount of CrO42- ions removed by all mixes increases gradually and continuously during the first 6 hours of mixing and nearly 80% of the chromate ions were removed during this time interval. From 6-24 hours, a gradual remove was noticed and an equilibrium stage was reached after 24 hours of the mixing process. For E1 and E2 mixes, nearly all the chromate ions present in CaO + Al2(SO4)3 suspensions are removed by ettringite. These indicates the success of the removal of these anions by the suspension products. For the rest of the mixes, nearly 90% of the chromate ions were removed by the suspension products. Also, we can note that the amount removed of CrO42- ions the suspension products increased by increasing the initial concentration of the CrO42- ions present in the suspensions. By considering that the ettringite is the main suspension product (as we show in the later sections), we can concluded that overcrowding in the chromate ions occurred by increasing its concentration, which increases its diffusion and rate of exchange inside ettringite crystals. However, from the obtained data all the studied mixes showed high removal of CrO42-, which related to the exchange of the sulfate ions by the chromate ions in the ettringite crystal. The uptake affinity of the ettringite phase toward the chromate ions is greater than other products like gypsum and calcite. This high uptake affinity of ettringite is related to the presence of three exchangeable sites in the structure of the ettringite.
Conclusion
1 - The products of the suspension of CaO and Al2(SO4)3 with molar ratio Ca/Al = 6 is the ettringite, with minor amounts of gypsum and calcite .
2 - According to the X-ray results and SEM micrographs, presence of chromate ions in the suspension results to slight decrease in the degree of the crystallinity and the size of the formed ettringite crystals.
3 - DTA results showed that the presence of chromate ions in that suspension decreases the rate of the reaction and the formation of the products.
4 - There is high removal of the chromate ions by CaO-Al2(SO4)3 suspension products, which is related to substitution of chromate ions in the crystal structure of ettringite.
References
1. James M. Tinjum, Craig H. Benson, Tuncer B. Edil. Mobilization of Cr(VI) from chromite ore processing residue through acid treatment. Science of The Total Environment ,Volume 391, Issue 1,2008, 13–25.
2. M. Schneider, K. Lipus. Low-chromate cements for improved industrial safety. ZKG International, 2002,6,55.
3- M. Magistri, D. Padovani. Chromate reducing agents. International Cement Review, 2005.
4. Takehiro M. Hydration and Setting of Portland Cement Added with Calcium Aluminate-Based Setters. Doctoral dissertation of Yamaguchi University, 2000, 90.
5. Bannister F.A., Hey M., Bernal J.D. Ettringite from Scawt Hill. Co. Antrim. Miner. Mag. 1936, 24, 324–329.
6. Deb S.K., Manghnani M.H., Ross K. et al. Raman Scattering and X-ray Diffraction Study of the Thermal Decomposition of an Ettringite-Group Crystal. Phys. Chem. Miner. 2003, 30, 31–38.
7. Myneni S.C.B., Traina S.J., Logan T.J. Ettringite Solubility and Geochemistry of the Ca(OH)₂–Al₂(SO₄)₃–H₂O System at 1 atm Pressure and 298 K. Chem. Geol. 1998, 148, –19.
8. Satoh K., Morinaga H., Tokumitu T. et al. Removement of Fluoride Ion by Ettringite. Abstr. 18th Fall Meeting of the Ceramic Society of Japan. 2005, 168.
9. Clark B.A., Brown P.W. The formation of calcium sulfoaluminate hydrate compounds, Part I. Cement Concrete Res.,1999, 29 1943–1948.
10. H. Poellman, S. Auer, H.J. Kuzel, R. Wenda, Solid solution of ettringites: Part II. Incorporation of B(OH)₄⁻ and CrO₄²⁻ in Ca₆Al₂O₆(SO₄)³· 32 H₂O. Cem. Concr. Res., 23 (1993), pp. 422–430.
11. M. Zhang. Incorporation of oxyanionic B, Cr, Mo, and Se into hydrocalumite and ettringite: Application to cementitious systems. Dissertation, University of Waterloo, Waterloo, Ontario, Canada, 1995, 171 pp.
12. R. Berardi, R. Cioffo, L. Santoro, Matrix stability and leaching behaviour in ettringite-based stabilization systems doped with heavy metals. Waste Management, 17 (8) (1997), pp. 535–540, 9-11.
13. Gabrisová A., Havlica J. Stability of Calcium Sulphoaluminate Hydrates in Water Solutions with Various pH Values. Cem. Concr. Res. 1991, 21, 1023–1027.
14. Barnett, S. J., Adam, C. D., & Jackson, A. R. W. (2001). An XRPD profile fitting investigation of the solid solution between ettringite, Ca₆Al₂(SO₄)₃(OH)₁₂· 26 H₂O, and carbonate ettringite, Ca₆Al₂(CO₃)₃(OH)₁₂· 26 H₂O. Cement and Concrete Research, 31(1), 13-17.
15. Shimada Y., Young J.F. Thermal Stability of Ettringite in Alkaline Solutions at 80°C. Cem. Concr. Res., 2004, 34, 2261–2268.
16. Chaipanich A, Nochaiya T. Thermal analysis and microstructure of Portland cement–fly ash–silica fume pastes. J Therm Anal Calorim. 2010; 99:487–93.
17. Amin MS, Abo-El-Enein SA, Abdel Rahman A, Khaled AA. Artificial pozzolanic cement pastes containing burnt clay with and without silica fume: physicochemical, microstructural and thermal characteristics. J Therm Anal Calorim. 2012; 107,1105–15.
18. Nochaiya T, Wongkeo W, Pimraksa K, Chaipanich A. Microstructural, physical, and thermal analysis of Portland cement–fly ash–calcium hydroxide blended pastes. J Therm Anal Calorim. 2010; 100: 101–8.
Cite this paper
APA
AbuAlola, K. A. A. (2013). Behavior of Chromate Ions in CaO-Al₂(SO₄)₃ Suspension. Open Science Repository Chemistry, Online(open-access), e70081936. doi:10.7392/Chemistry.70081936
MLA
AbuAlola, Khulood A. A. “Behavior of Chromate Ions in CaO-Al₂(SO₄)₃ Suspension.” Open Science Repository Chemistry Online.open-access (2013): e70081936.
Chicago
AbuAlola, Khulood A. A. “Behavior of Chromate Ions in CaO-Al₂(SO₄)₃ Suspension.” Open Science Repository Chemistry Online, no. open-access (March 6, 2013): e70081936. http://www.open-science-repository.com/behavior-of-chromate-ions-in-cao-al2so43-suspension.html.
Harvard
AbuAlola, K.A.A., 2013. Behavior of Chromate Ions in CaO-Al₂(SO₄)₃ Suspension. Open Science Repository Chemistry, Online(open-access), p.e70081936. Available at: http://www.open-science-repository.com/behavior-of-chromate-ions-in-cao-al2so43-suspension.html.
Science
1. K. A. A. AbuAlola, Behavior of Chromate Ions in CaO-Al₂(SO₄)₃ Suspension, Open Science Repository Chemistry Online, e70081936 (2013).
Nature
1. AbuAlola, K. A. A. Behavior of Chromate Ions in CaO-Al₂(SO₄)₃ Suspension. Open Science Repository Chemistry Online, e70081936 (2013).
doi
Research registered in the DOI resolution system as: 10.7392/Chemistry.70081936.

This work is licensed under a Creative Commons Attribution 3.0 Unported License.