Open Science Repository Chemistry
doi: 10.7392/Chemistry.70081948
Distinct Antimicrobial Effects of Synthesized ZnS Nanoparticles Against Twelve Pathogenic Bacterial Strains
Sutapa Ganguly [1], Sukhen Das [1], Sujata Ghosh Dastidar [2]
[1] Department of Physics, Jadavpur University, Kolkata- 700 032, India.
[2] Department of Microbiology, Herbicure Healthcare Bio-Herbal Research Foundation, Kolkata- 700 104, India.
Abstract
The zinc sulphide nanoparticles were synthesized by simple aqueous chemical reaction of zinc chloride and sodium sulphide in aqueous solution. The main advantage of the ZnS nanoparticles of diameter 29 nm is that the sample is prepared by using of non-toxic precursors in a cost effective and eco-friendly way. The structural, morphological and chemical composition of the nanoparticles have been investigated by X-Ray Diffraction(XRD),Scanning Electron Microscopy(SEM) with energy dispersion X-ray fluorescence spectroscopy (EDAX) and Fourier transform infrared (FTIR) spectroscopy. The antimicrobial effects of the zinc sulphide (ZnS) nanocrystals were studied by well diffusion technique against twelve pathogenic bacterial strains. ZnS showed antimicrobial activity against both gram positive and gram negative strains except Shigella sonnei.
Keywords: zinc sulfide, nanoparticles, aqueous chemical synthesis, antimicrobial.
Citation: Ganguly, S., Das, S., & Dastidar, S. G. (2013). Distinct Antimicrobial Effects of Synthesized ZnS Nanoparticles Against Twelve Pathogenic Bacterial Strains. Open Science Repository Chemistry, Online(open-access), e70081948. doi:10.7392/Chemistry.70081948
Received: February 25, 2013
Published: March 29, 2013
Copyright: © 2013 Ganguly, S., Das, S., & GhoshDastidar, S. Creative Commons Attribution 3.0 Unported License.
Contact: [email protected]
Introduction
Extensive and often indiscriminate application of these agents for many years have resulted in an explosion of multiple drug resistant pathogens throughout the world. Thus, there is an urgent need to identify and develop new antimicrobial compounds, either natural or synthetic, to offer appropriate and efficient therapy for various types of infections. Interests on nanoparticles have been generated in recent years due to their simple structure, characteristic physical, chemical and biological properties that are usually distinctly different from those of the bulk materials. Intensive experiments and studies have revealed that the nanoparticles of magnesium oxide, calcium oxide and zinc oxide1,2 possess potent antimicrobial property when tested against various Gram positive and Gram negative organisms. Zinc sulphide is a simple inorganic compound known for its practical applications in photoconductors, solar cells, field effect transistors, sensors transducers , optical coatings and light emitting materials3.It may be pointed out here that simple inorganic substances as antimicrobial agents may prove to be advantageous as they contain mineral substances essential for human consumption and may exhibit powerful action even when administered in small amounts.In view of the information on presence of antibacterial action in nanoparticles of MgO, CaO and ZnO, nanoparticles of ZnS was prepared in our laboratory and was evaluated for the antimicrobial potentiality along with that of imatinib singly and by combination of the two agents.
Materials and methods
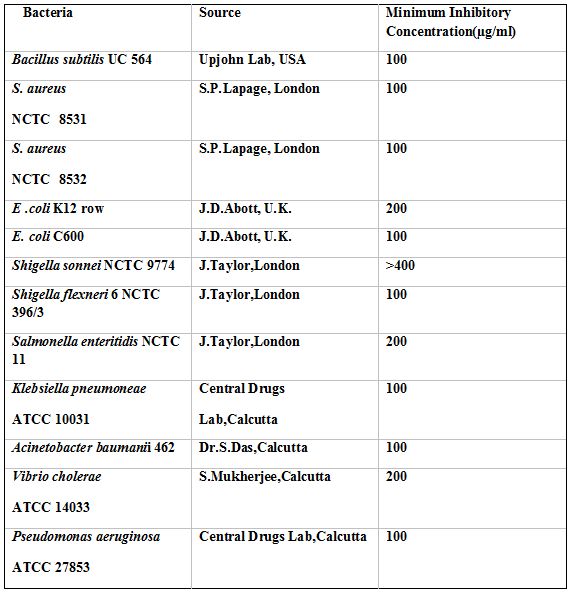
Bacteria: A total of 12 pathogenic bacteria belonging to 8 genera comprising 9 Gram negative and 3 Gram positive strains were tested.These were of human origin, identified as described by Barrow and Feltham4 and preserved in freeze dried state.Chemical compounds: Analar ZnCl2 and Na2S were purchased from Merck, Germany, these were allowed to react to produce ZnS nanoparticles.
Media: Liquid media used for the study were nutrient broth (NB,Oxoid) and Mueller Hinton broth (MHB, Oxoid): solid media were nutrient agar (NA,Oxoid) and Mueller Hinton agar (MHA,Oxoid)
Method of preparation of zinc sulphide nanoparticles
Synthesis of ZnS nanoparticles was carried out by aqueous chemical method using ZnCl2 and Na2S as source materials. All the reagents were of analytical grade and used without further purification. The entire process was carried out in distilled water for its inherent advantages of being simple and environment friendly. All steps of the synthesis were performed at low temperature and ambient conditions. In a typical preparation solution of 1M Na2S was added drop by drop to 1M ZnCl2 solution which was kept on stirring using a magnetic stirrer at 70 oC for 2 hours, this resulted in formation of ZnS nanocolloid. The nanoparticles were collected by centrifugation at 2000 rpm for 15 minutes and further purification was made in ultrasonic bath. The resultant product was finally dried at 120 ºC for 2 h.
Characterization of ZnS nanoparticles
The prepared sample was subjected to characterization by X-ray diffraction (XRD) (Model D8, Bruker AXS) to determine the phase purity and average particle size of the sample, using CuKα radiation at 1.5409Å (2Ө = 100-700, scan speed = 0.2 s/step, increment = 0.02, operating voltage = 40 kV and operating current = 40 mA). The nanophase was identified by comparing peak positions and intensities (finger print method).
To determine the structural features of all the samples, Fourier transform infrared (FTIR) spectroscopy was carried out using an FTIR spectrometer (FTIR-8400s, Shimadzu), with 150 scans for wave numbers ranging from 400-4000 cm-1 and resolution 4 cm-1. The KBr pellet method was used to prepare the samples5.
To investigate the morphological structure of sample surfaces, surface textures were examined by field emission scanning electron micrography (FESEM) and energy dispersion X-ray fluorescence spectroscopy (EDAX) (JSM6700F JEOL LTD, Tokyo, Japan), was also carried out to ascertain the composition.
X-ray diffraction (XRD) analysis
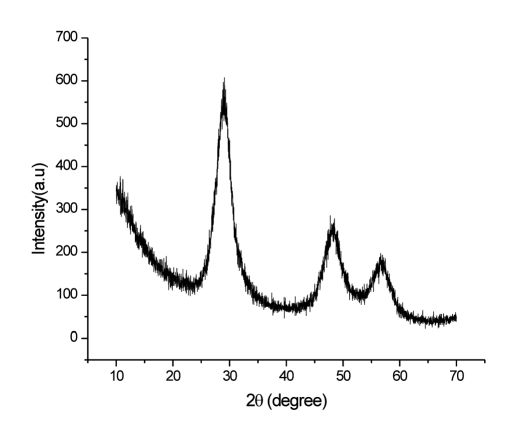
From the XRD results as shown in figure 1, it is clear that the pure ZnS nanoparticles have been obtained in powder form. The broadened peaks in the XRD pattern indicate the formation of ZnS nanocrystals with small crystallites. The three diffraction peaks at 2θ values of 28.9780, 47.620, 56.650 correspond to the (111), (220) and (311) diffraction planes, respectively of the spherical nanocrystalline structure of ZnS were observed. These values were very close to those reported by Jia Xiang Yang et.al.6.
The average crystallite size (D) was calculated from the full-width at half-maximum (FWHM) of the most intense peak of the (111) plane of ZnS nanoparticles using the Debye-Scherrer formula for spherical particles [Eq. (1)].
D = 0.89λ/ (β cos θ) (1)
Where λ is the wavelength (Cu Kα), β is the full width at the half-maximum of the ZnS nanoparticles and θ is the diffraction angle.
From this equation the average particle size was estimated to be 29 nm which was also supported from FESEM.
Fourier transforms infrared (FTIR) studies
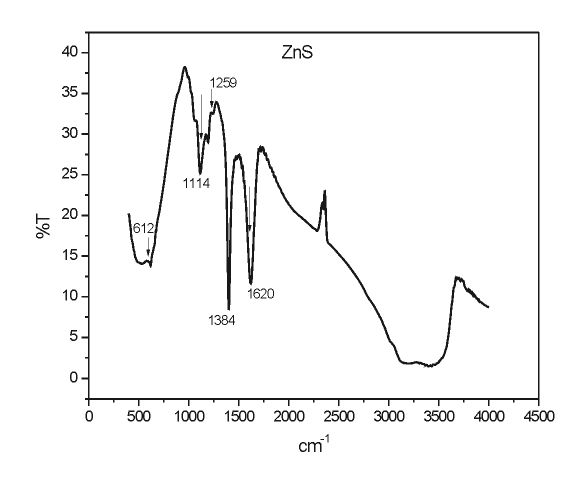
Figure 2 shows the FTIR spectrum of the ZnS nanoparticles. The spectra exhibit strong bands appearing in the 1114, 1259, 1384 & 3200–2900 cm-1 correspond to ZnS nanoparticles5. The peaks at 612 cm-1 is assigned to the ZnS band (i.e., corresponding to sulphides)6,7.The O–H bending region due to absorbed water appears at 1620 cm-1. The stretch vibration adsorption of ZnO at 420−460 cm−1 is not detected which indicates that ZnS was not oxidized to ZnO during the preparation as reported by She Yuan-yuan et.al8.
FESEM analysis and EDAX study
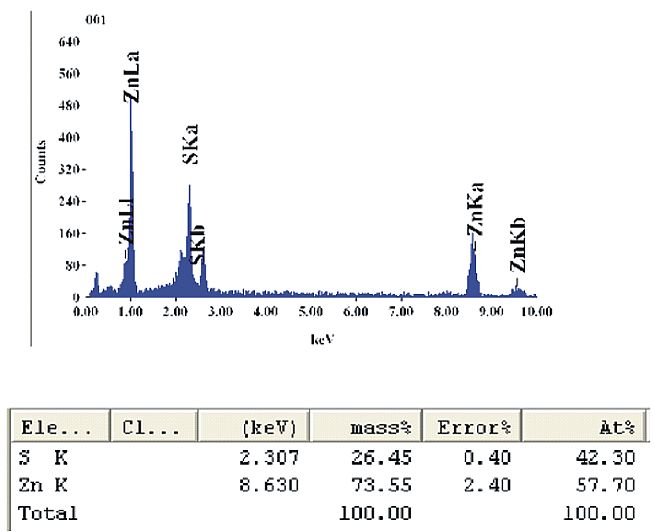
Figures 3 and 4 show the FESEM/EDAX results of as prepared ZnS nanoparticles. It is seen that the ZnS nanoparticles are homogenously dispersed and almost spherically shaped with an average diameter of about 29 nm. From the EDAX result we get the composition of the prepared sample i.e. about 73.55% of Zn+ ion and about 26.45% S ion by mass are present in the sample.
Preparation of zinc sulphide nanoparticle solution
To prepare ZnS nanoparticle solution 0.01g of the synthesized ZnS nanoparticles were dissolved in 10 microlitre of dilute HCl and the volume was made upto 10ml with sterile distilled water. The final concentration of ZnS nanoparticles in the solution was 1µg/ml.This solution was applied in the wells bored in the agar plates for the study of antimicrobial activity alone and in combination with Imatinib. A control solution was prepared which contained 1-2 drops of dilute HCl in 10ml of water and did not contain ZnS nanoparticles.
In vitro tests for determination of minimum inhibitory concentration (MIC) of ZnS nanoparticles
The Gram negative bacteria were grown in Mueller–Hington broth and the Gram positive ones in nutrient broth for 18h to obtain optimum growth.
An aqueous 10mg/ml stock solution of ZnS nanoparticles was prepared in sterile distilled water. This was added to molten nutrient agar at 50ºC in such a manner that the final concentrations were 0(control),100,200,300,400 µg/ml , thoroughly mixed, final pH adjusted to 7.2 to 7.4 and poured into sterile Petri dishes .The inocula consisted of suitably diluted 18h broth culture of bacterium. The MIC of ZnS nanoparticles was determined by spot inoculating one 2mm {internal diameter) loopful of a culture containing ca.105 colony forming units (CFU), on the plates following the guidelines of CLSI. The plates were incubated at 37 oC. Growth was recorded at 18h as well as up to 72h .
Determination of antimicrobial action of ZnS nanoparticles by well diffusion assay
The in vitro effect of the agents was determined by well diffusion technique as described by Miles and Amyes9.The wells were cut with the help of sterile cork borers on the agar surface at suitable distances apart, so that the respective concentrations of the agent would not diffuse into one another to produce a continuous range of concentrations in the initial period of inhibition. This was done by initial well sensitivity test of a microorganism with respect to a particular concentration of an agent and determining the diameters of zone of inhibition.
Antibacterial activity of ZnS nanoparticles by in vitro screening
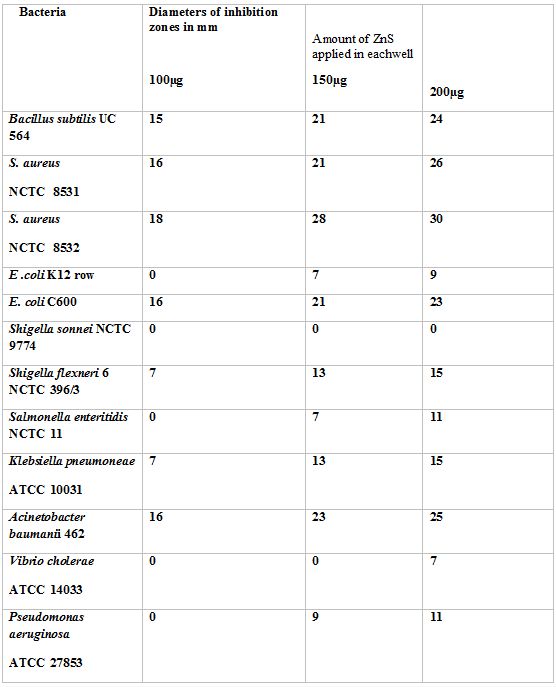
The MIC of ZnS nanoparticles against different bacteria as observed by spot inoculation method is presentated in Table 1. This shows that B. subtilis UC 564, S. aureus 8531, 8532, E. coli C600, Sh. Flexneri 6, K. pneumonia 10031, A. baumanii 462 and P. aeruginosa 27853 were inhibited at 100µg/ml of ZnS; E. coli K12 Row, S. enteric 11 and V. cholerae 14033 were inhibited at 200µg/ml of ZnS; Sh. sonnei 9774 remained totally resistant to ZnS.
The nanoparticles of ZnS produced inhibition zones around the wells that varied from 7 mm to 18 mm when the amount of ZnS was 100 ug per well (Table 2).The diameters of inihibitory circles increased in size as the amount of ZnS was increased (Table 2).The The greater sensitivity of Gram positive organisms by ZnS was further confirmed by this test.
Discussion
The present study clearly indicates that ZnS nanostructures could be synthesized by a simple aqueous chemical method using pure aqueous route resulting in primary particle sizes of 29 nm. This particle size was calculated from Debye –Scherrer formula. FESEM image was used to study the morphology of the synthesized nanoparticles. FTIR spectra showed the possible stretching and bending modes of the ZnS nanoparticles. These ZnS nanoparticles synthesized by us showed significant antimicrobial activity when tested against pathogenic bacterial strains. While sensitive bacterial strains included B. subtilis UC 564, A. baumanii 462, E. coli C600, K. pneumoniae atcc 10031, S. aureus 8531 and 8532 and P. aeruginosa ATCC 27853. It was found to be less active against Sh. sonnei 9774, V. cholerae atcc 14033and E. coli K12 Row. It may be pointed out here that ZnS nanoparticles demonstrated a pronounced inhibitory action against S.aureus 8531, an organism which is known to be multidrug sensitive10. ZnS nanoparticles were found to be bacteriostatic in vitro against both Gram positive and Gram negative bacteria.These results reveal that ZnS Nanoparticles possess significant antimicrobial activity which may be enhanced in combination with other antimicrobial agents. Further studies are in progress to explore the possibility of their application in routine therapy against infections of animals.
Acknowledgements
We are grateful to UGC (University Grants Commission) Government of India, for financial assistance.Figures and tables
References
1. Padmavati N & Vijayaraghavan R. (2008). Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci.Technol.Adv.Mater. 9 1468.
2.
Nair S, Sashidharan A, Divyarani V V, Menon D, Nair S, Manzoor K,
Raina S. (2009). Role of size scale of ZnO nanoparticles and
microparticles on toxicity towards bacteria and osteoblast
cancer cells. J.Mat.Sc:Mater.
Med.
20, 235.
3.
John R & Sasiflorence S. (2010). Optical,structural and morphological
studies of bean like ZnS nanostructures by aqueous chemical method,
Chalcogenide
Let. 7 269.
4.
Barrow G I & Feltham R K A. (1993). Cowan
and Steels manual for the identification of medical
Bacteria. Cambridge
university press,Cambridge,UK, 329.
5. Criado, M., Fernández-Jiménez, A., & Palomo, A. (2007). Alkali activation of fly ash: Effect of the SiO2/Na2O ratio Part I: FTIR study. Microporous and Mesoporous Materials, 106, 180-191.
6.
Yang J X, Wang S M, Zhao X, Tian Y P, Zhang S Y, Jin B K, Hao X
P, Xu X Y, Tao X T, Jiang M H. (2008). Preparation and characterization of
ZnS nanocrystal from Zn(II) coordination polymer and ionic liquid. J.Crystal
Growth, 310 4358.
7. Rema Devi B S, Raveendran R, Vaidyan A V. (2007) Synthesis and characterization of Mn+2 doped ZnS nanoparticles. Pramana-J.Phy. 68, 679.
8. Yuan-Yuan S, Juan Y, Ke-qiang Q. (2011). Synthesis of ZnS nanoparticles by solid liquid chemical reaction with ZnO and Na2S under ultrasonic bath. Transactions Of Non Ferrous Metals Society Of China. 20, 211.
9.
Miles & Amyes. (1996). Laboratory
control of antimicrobial therapy. 225.
10.
Dasgupta A,
Dastidar SG, Shiratki Y, Motohashi N. (2008). Antibacterial activity of
artificial phenothiazines and isoflavones from plants. In:
Bioactive Heterocycles
VI.
15, 67-132
Cite this paper
APA
Ganguly, S., Das, S., & Dastidar, S. G. (2013). Distinct Antimicrobial Effects of Synthesized ZnS Nanoparticles Against Twelve Pathogenic Bacterial Strains. Open Science Repository Chemistry, Online(open-access), e70081948. doi:10.7392/Chemistry.70081948
MLA
Ganguly, Sutapa, Sukhen Das, and Sujata Ghosh Dastidar. “Distinct Antimicrobial Effects of Synthesized ZnS Nanoparticles Against Twelve Pathogenic Bacterial Strains.” Open Science Repository Chemistry Online.open-access (2013): e70081948.
Chicago
Ganguly, Sutapa, Sukhen Das, and Sujata Ghosh Dastidar. “Distinct Antimicrobial Effects of Synthesized ZnS Nanoparticles Against Twelve Pathogenic Bacterial Strains.” Open Science Repository Chemistry Online, no. open-access (March 29, 2013): e70081948. http://www.open-science-repository.com/distinct-antimicrobial-effects-of-synthesized-zns-nanoparticles-against-twelve-pathogenic-bacterial.html.
Harvard
Ganguly, S., Das, S. & Dastidar, S.G., 2013. Distinct Antimicrobial Effects of Synthesized ZnS Nanoparticles Against Twelve Pathogenic Bacterial Strains. Open Science Repository Chemistry, Online(open-access), p.e70081948. Available at: http://www.open-science-repository.com/distinct-antimicrobial-effects-of-synthesized-zns-nanoparticles-against-twelve-pathogenic-bacterial.html.
Science
1. S. Ganguly, S. Das, S. G. Dastidar, Distinct Antimicrobial Effects of Synthesized ZnS Nanoparticles Against Twelve Pathogenic Bacterial Strains, Open Science Repository Chemistry Online, e70081948 (2013).
Nature
1. Ganguly, S., Das, S. & Dastidar, S. G. Distinct Antimicrobial Effects of Synthesized ZnS Nanoparticles Against Twelve Pathogenic Bacterial Strains. Open Science Repository Chemistry Online, e70081948 (2013).
doi
Research registered in the DOI resolution system as: 10.7392/Chemistry.70081948.

This work is licensed under a Creative Commons Attribution 3.0 Unported License.